Abstract
Background:
Ordinary pressure dressing for hemostasis after cardiac catheterization is time consuming and might cause some problems, such as pain or loss of blood, which has to be controlled, particularly in pediatric patient. Using Celox® (chitosan) powder dressing might cause quicker initial hemostasis.Methods:
In this prospective study, we assessed Celox® powder among patients in hospitals affiliated with Shiraz University of Medical Sciences, from November 2017 to February 2018. The patients were stratified in two groups. The case group included patients for whom Celox® powder was used along with sterile gauze pressure at the puncture site to achieve hemostasis, and the control group those in whom hemostasis was achieved by standard sterile gauze pressure method.Results:
Sixty patients under 16 years of age with congenital heart diseases were evaluated and underwent cardiac catheterization. We stratified the patients in two groups called case (30 patients) and control group (30 patients). Considering both arterial and venous initial hemostasis, in the case group, the minimum and maximum, median and mean coagulation time were less than those in the control group; however, the initial hemostasis was statistically significant only in venipuncture site. Also, the venous coagulation time was shorter among the patients weighing less than 10 kilograms in comparison to those with higher weight. In the case group, using Celox® stirred hemostasis toward the lower percentiles, but based on 50th percentile, the distribution in each group was identical.Conclusions:
Celox® powder dressing in children led to reduced coagulation time in venipuncture site, and we might recommend utilizing this type of dressing for venous hemostasis in children after venipuncture.Keywords
Cardiac Catheterization Celox Chitosan Hemostasis Pediatrics
1. Background
Bleeding control techniques have remained unchanged. Strip bandage, direct manual pressure, pressure dressing, tourniquet and direct clamp are common methods that are used to achieve hemostasis. Although studies have continuously attempted to improve hemostatics, substituted techniques to control bleeding were only found in the last decade.
An ideal local hemostatic medication must have a considerable homeostatic effect, low tissue reaction, and easy sterilization method as well as being bioresorbable, of low cost and usable for specific needs (1).
Increasing knowledge on the importance of rapid control of bleeding in military operations has led to methods of reducing bleeding time in puncture sites. For this reason, some local hemostatic agents were evaluated by military. These agents include HemCon (strips containing chitosan) or QuikClot (Zeolite powder dressings), chitosan-based hemostatics (2, 3), porous polyethylene fiber-based hemostatics, hydrogels (4), fibrinogen/thrombin-impregnated matrices and kaolin-impregnated dressing (5, 6).
Using sandbag and pressure dressings might cause severe pain in the catheter insertion site and increase the probability of bleeding or hematoma as well as increase hospitalization period due to the prolonged absolute rest, while other techniques with less negative effects are quite more effective to ease patients’ pain and fatigue (7).
Application of pressure dressings is time consuming, particularly after cardiac angiography and might cause trouble for patients caused by prolonged absolute rest, such as pulmonary failure to fill up completely with air, secretion accumulation, workload increase of the heart followed by increased cardiac work and sense of dependency (8).
Celox, approved by FDA in 2006, is made of Chitosan granules, and shellfish endoskeleton, an inert non-biodegradable kaolin mineral powder, with weak hemostatic power, which triggers the coagulation cascade (9-12). The initial studies were controversial regarding chitosan dressing effects on decreasing blood loss and mortality, in comparison with standard gauze bandage (10, 13).
Bleeding control techniques in children are important, since they are less obedient than adults, and also bleeding hazards are more dangerous in pediatric patients due to patients’ lower total blood volume. Hence, small amount of bleeding can cause complication, which might be hazardous. Also, hospitalization threats for this age group are more serious than adults because of their weaker immune system which has to be considered (14).
Several papers have evaluated Chitosan role in bleeding control caused by trauma, vascular access for hemodialysis and after cardiac catheterization (10, 15-17). However, the target population in all these papers were adults, while children were not included. There are various differences in functionality of the coagulation system, plasma constituents and erythrocyte level between adults and children, and some differences between functionality of Celox® powder in different age groups might exist (14). Hence, it seems necessary to evaluate this novel bleeding control method in children.
2. Objectives
The purpose of this study was to compare the effect of standard gauze pressure with and without Celox to stop bleeding and hemostasis of the cardiac catheterization site among pediatric patients. This investigation was carried out with the hope of improving hemostasis and reduced hospitalization. Our hypothesis was that using sterile gauze with Celox® powder might be more effective to stop the bleeding in comparison with application of just standard gauze.
3. Methods
In this prospective study, pediatric patients with congenital heart disease who had undergone cardiac catheterization in hospitals affiliated to Shiraz University of Medical Sciences, Shiraz, Iran, were evaluated from November 2017 to February 2018. The current research was approved by the local Ethics committee of Shiraz University of Medical Sciences (code: 1396-01-01-14802).
The research goals were explained for the patients or guardians and their written consent obtained.
The inclusion criteria were children under the age of 16 with congenital heart disease who were under diagnostic or therapeutic cardiac catheterization. The exclusion criteria were, known systemic or coagulation diseases, low platelet count, prolonged partial thrombin and prothrombin time, and international normalized ratio.
Besides, patients who received aspirin, warfarin or other antiplatelet or anticoagulant medications before the procedures and patents with unpredictable polycythemia were excluded.
Initially, medical history was obtained by asking questions from the patients or parents and by checking the patients’ medical record. Written informed consent was obtained form each patient or parents/guardian.
Local anesthesia was performed by injection of 1% lidocaine without epinephrine, and arterial or venous access was performed from right or left femoral site percutaneously, using the Seldinger method.
All patients received 100 Unit/kg heparin after insertion of the sheaths, and no patient received protamine for converting the effect of heparin.
After catheterization, the sheaths were removed and patients were stratified into two groups. The case group included patients in whom 2 grams of Celox® powder (MedTrade Products Ltd, Cheshire, UK) were applied along with sterile gauze pressure at the puncture site to achieve hemostasis. Patients of the control group received solely standard sterile gauze pressure to stop the bleeding.
The two groups were matched for age and gender based on stratified randomization. The research was performed as case-control study without using placebo.
3.1. Monitoring and Treatment
Axillary temperature was monitored and kept between 37°C and 37.5°C and blood pressure was maintained in the normal range according to patient’s age.
Two thin plies of a sterile gauze were pressed on the wound briskly until bleeding stopped and then Celox® powder was applied to the site under the gauze.
Stopped bleeding time was recorded by a stop-watch. Other variables such as right or left catheterization, diagnostic or interventional procedure, sheath size, vascular access site as well as anticoagulant medications were also recorded. After data completion, results were analyzed clinically and statistically.
The maximum bleeding control was defined as the time in which no active bleeding was observed by removing the pressure from the access point.
In all patients, bleeding control was carried out in the first three minutes, by exerting pressure on the bleeding site. If there was still an active bleeding after three minutes, pressure was continued and every 2 minutes, the pressure was reduced slightly until complete bleeding control was achieved. If there was a complete active bleeding after three minutes, another three minutes of pressure was applied and then the puncture site was checked again.
In data analysis, initially database normalization was evaluated using Kolmogorov-Smirnov test corrected by Lilliefors in which for normal data, appropriate parametric methods such as Student t-test and for non-normal data, the Mann-Whitney test was used. For analysis of the data with a nominal scale, chi-Squared test was applied and where more than 20% of the expected abundance was less than 5, Fisher’s Exact Test was used. Data were analyzed with SPSS version 20 software. Significance level was set at ≤ 5%.
4. Results
In this survey 60 patients were evaluated. Case and control group each consisted of 30 patients. In the case group there were 18 boys and 12 girls and in the control group 16 boys and 14 girls with an average age of 33.7 ± 6.9 and 30 ± 7.1 months, respectively. The results are presented in Table 1. There were no differences between the variables age, weight, size of the needles used for venipuncture and the duration of the procedures in the patients and controls.
| Variables | Case (N = 35) | Control (N = 30) | P Value |
|---|---|---|---|
| Sex | 0.399 | ||
| Male | 18 | 16 | |
| Female | 12 | 14 | |
| Age, mo | 31.70 ± 6.92 | 32.41 ± 7.10 | 0.696 |
| Weight, kg | 11.84 ± 1.48 | 12.17 ± 1.36 | 0.3722 |
| Procedure duration, min | 59.43 ± 4.16 | 58.66 ± 2.37 | 0.3820 |
| Cyanotic heart disease, n | 8 | 7 | 0.902 |
| Acyanotic heart disease, n | 22 | 23 | |
| Femoral vein puncture, n | 28 | 26 | 0.695 |
| Femoral artery puncture, n | 20 | 23 | 0.512 |
| Needle or introducer size, F | 7.13 ± 1.31 | 7.21 ± 1.56 | 0.830 |
| Hemoglobin, g/dL | 13.20 ± 2.65 | 12.95 ± 1.99 | 0.681 |
| Platelet count | 205000 ± 1.33 | 210000 ± 1.21 | 0.394 |
| Prothrombin time | 15.41 ± 2.11 | 14.91 ± 2.45 | 0.400 |
| Partial thrombin time | 33.41 ± 4.71 | 32.84 ± 5.34 | 0.663 |
| International normalizing ratio | 1.13 ± 0.23 | 1.17 ± 0.31 | 0.572 |
In both arterial and venous venipuncture sites, the minimum, maximum, median and mean initial hemostasis times in the case group were less than those in the control group, though the difference was not statistically significant (P = 0.769) (Table 2), while for coagulation time the differences were significant (Figure 1 and Table 3).
Celox effect on the artery and vein hemostasis among the case and control groups
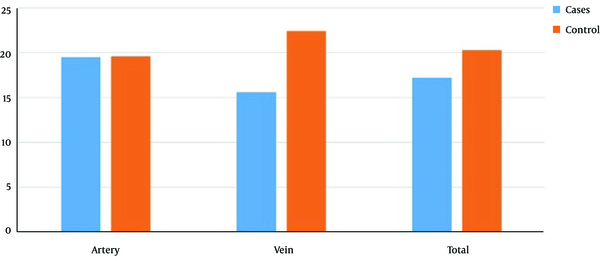
Duration of the Initial Hemostasis in Arteries and Veins of the Case and Control Groups
| Group | Minimum | Maximum | Median | Mean ± SD | P Value |
|---|---|---|---|---|---|
| Case, min | 4 | 30 | 15 | 17.2 ± 6.7 | 0.769 |
| Control, min | 10 | 60 | 18.5 | 20.3 ± 13.6 |
Comparison of the Initial Hemostasis in Different Parameters of the case and Control Groupsa
| Independent Factor, Number | Initial Hemostasis | P Value | |
|---|---|---|---|
| Case Group | Control Group | ||
| Type of the heart disease | |||
| Cyanotic (n = 15) | 15.6 ± 8.2 | 17.2 ± 7.9 | 0.7 |
| Acyanotic (n = 45) | 17.6 ± 6.3 | 21.4 ± 15.2 | 0.2 |
| Gender | |||
| Male (n = 30) | 15.6 ± 6.3 | 19.6 ± 12.8 | 0.2 |
| Female (n = 30) | 19.3 ± 6.8 | 20.8 ± 14.6 | 0.7 |
| Age, mo | |||
| ≤ 12 (n = 36) | 16.1 ± 6.9 | 20.1 ± 12.8 | 0.2 |
| > 12 (n = 24) | 18.7 ± 6.5 | 20.5 ± 15.6 | 0.7 |
| Weight, kg | |||
| ≤ 10 (n = 27) | 15.4 ± 6 | 14.8 ± 5 | 0.7 |
| > 10 (n = 33) | 19.7 ± 7.1 | 23.4 ± 15.9 | 0.4 |
| Puncture site | |||
| Femoral artery (n = 43) | 19.5 ± 6 | 19.6 ± 12.7 | 0.2 |
| Femoral vein (n = 34) | 15.6 ± 6.8 | 22.4 ± 17 | 0.04 |
| Needle or introducer size, French | |||
| Size < 8 (n = 41) | 15.7 ± 5.3 | 19.2 ± 13 | 0.2 |
| Size ≥ 8 (n = 19) | 20 ± 8.4 | 22.5 ± 15.3 | 0.6 |
| Procedure duration | |||
| ≤ 60 min (n = 50) | 16.3 ± 6.7 | 17 ± 9.5 | 0.7 |
| > 60 min (n = 15) | 21 ± 5.4 | 27 ± 18.7 | 0.4 |
Considering these factors, Celox can reduce bleeding time of the venipuncture site.
Also, we compared the venous coagulation time among other variables and noticed the only variable with significant relation was weight, and the venous coagulation time was shorter in patients weighing less 10 kg in comparison with those weights more than 10 kg (15.25 ± 5.16 vs 19.04 ± 6.79; P value = 0.024).
Using Celox, derived hemostasis of the case group toward the lower percentiles (Table 4), although the distribution in each group based on 50th percentile was not significant (P = 0.6).
Comparison of the Hemostasis Time According to Different Percentiles Between the Patient and Control Groups
| Percentile | Initial Hemostasis (min) in Case Group | Initial Hemostasis (min) in Control Group |
|---|---|---|
| 25% | 13.71 | 14.27 |
| 50% | 16.42 | 17.32 |
| 75% | 21.75 | 23.06 |
| 90% | 27.50 | 40.03 |
5. Discussion
There are limited studies on Celox effect in children and none in those with congenital heart disease to enable us to compare our results on the effect of Celox® powder in children with those of other studies.
Celox® powder was approved by FDA in 2006. Initial researches showed that chitosan dressing can significantly decrease blood loss and mortality by increasing hemostasis, in comparison with standard gauze bandage (1).
According to the results, significant differences were observed when Celox was applied on venipuncture site (P = 0.04); however, we did not observe any significant hemostasis preference when using Celox on arterial puncture site for cardiac catheterization of congenital heart disease. And although Celox® powder decreased the initial hemostasis of arterial and venous puncture sites in all patients in our study, it was not statistically significant for arterial hemostasis (P = 0.2) (Table 3). Failure to improve arterial hemostasis might be due to the arterial jet pressure, which washes the powder from the puncture site.
Despite the usefulness of Celox on arterial and venous hemostasis in adults according to previous studies, the differences in the result of the current study might be due to variation in coagulation system and the functionality of the coagulation pathways in various age groups (18-20). In addition, the differences between plasma content, erythrocyte levels and their negative charge might be influential in obtaining different results (15).
Several papers have evaluated the role of chitosan in control of bleeding caused by trauma, vascular access for hemodialysis and after cardiac catheterization (4-9). Generally, the target population in all the aforementioned papers were adults, while children were not included.
In an investigation, 160 patients with organ trauma were divided into two groups of case and control. Hemostasis occurred within 5 minutes for 32.5 % of the control and 51.3 % of case gtoups. The authors concluded that gauze impregnated with Celox was able to achieve hemostasis in the injured organ quite faster than the common pressure dressing.
Another study showed that there was significant difference in hemostasis time, when using 0.5 - 1 gr of Celox® powder in comparison with common dressing. It was deduced that using Celox® powder can decrease hemostasis time in vascular access site for hemodialysis patients. Therefore, it was recommended for patients with delayed hemostasis in vascular access site (12).
According to the table 4, percentile distribution of the coagulation time among the control group in arteries and veins decreased, but the difference was not statistically significant.
Also, some researchers stated that Celox reduced recurrent bleeding to 0% while for common dressing this value was more than 17%. Furthermore, Celox rose hemostatic stability in all the cases while this rate for common dressing was about 50%. They recommended using Celox as a stable substituting technique to treat severe bleeding (13). In the current survey, recurrent bleeding was not observed in any of the case group members; hence, the impact of Celox was not evaluated.
In spite of the previous studies, one study did not observe any significant difference when using Celox in comparison with using standard gaze method, which might have been due to opaque dressing adhesives, large dressing area in catheter insertion site and disorders in the value of bleeding evaluation (14).
Among studies in which target group were children, a study can be noticed, which was performed on 5 children with average age of 2 years who were suffering from severe coagulation disorders. To treat external bleeding in these children, dressing containing chitosan was used during 6 bleeding episodes. This dressing was used as the first therapeutic selection or as a second selection when the standard treatment was not effective. In 4 out of 6 bleeding episodes, chitosan dressing stopped the bleeding in the first few seconds. Recurrent bleeding and allergic skin reaction were not recorded after dressing removal, and they reported applying chitosan dressing as a good choice for external bleeding in children with severe coagulopathy disorders (15). The mentioned study was a case report and it was not evaluated as a case-control research, while our study did not assess bleeding in serious coagulation disorders (which was the exclusion criterion). Therefore, clarifying these disorders goes beyond the scope of this paper.
5.1. Limitations
The most important limitation in this study, was not having the ability of using double-blind trial for our case and control groups, and measuring the exact time of bleeding and hemostasis was the other limitation. This research was done with great effort to minimize the impact of these factors, by using a similar protocol to control the ongoing bleeding in both groups.
5.2. Conclusions
Celox® powder dressing in children reduced the time of coagulation in venipuncture area, even though it was not statistically significant in comparison with pressure dressing in arterial puncture sites. Hence it might be recommended to utilize this kind of dressing for venous hemostasis in children after cardiac catheterization and angiography, but more studies are warranted with larger population to assess the effectiveness of Celox in catheterization of children.
Acknowledgements
References
-
1.
Pogorielov M, Kalinkevich O, Deineka V, Garbuzova V, Solodovnik A, Kalinkevich A, et al. Haemostatic chitosan coated gauze: in vitro interaction with human blood and in-vivo effectiveness. Biomater Res. 2015;19:22. [PubMed ID: 26528399]. [PubMed Central ID: PMC4629397]. https://doi.org/10.1186/s40824-015-0044-0.
-
2.
Morrison JJ, Mountain AJ, Galbraith KA, Clasper JC. Penetrating pelvic battlefield trauma: internal use of chitosan-based haemostatic dressings. Injury. 2010;41(2):239-41. [PubMed ID: 19913787]. https://doi.org/10.1016/j.injury.2009.10.023.
-
3.
Alam HB, Chen Z, Jaskille A, Querol RI, Koustova E, Inocencio R, et al. Application of a zeolite hemostatic agent achieves 100% survival in a lethal model of complex groin injury in Swine. J Trauma. 2004;56(5):974-83. [PubMed ID: 15179235].
-
4.
Hamedi H, Moradi S, Hudson SM, Tonelli AE. Chitosan based hydrogels and their applications for drug delivery in wound dressings: A review. Carbohydr Polym. 2018;199:445-60. [PubMed ID: 30143150]. https://doi.org/10.1016/j.carbpol.2018.06.114.
-
5.
Kheirabadi BS, Scherer MR, Estep JS, Dubick MA, Holcomb JB. Determination of efficacy of new hemostatic dressings in a model of extremity arterial hemorrhage in swine. J Trauma. 2009;67(3):450-9. discussion 459-60. [PubMed ID: 19741385]. https://doi.org/10.1097/TA.0b013e3181ac0c99.
-
6.
Li H, Wang L, Alwaal A, Lee YC, Reed-Maldonado A, Spangler TA, et al. Comparison of Topical Hemostatic Agents in a Swine Model of Extremity Arterial Hemorrhage: BloodSTOP iX Battle Matrix vs. QuikClot Combat Gauze. Int J Mol Sci. 2016;17(4):545. [PubMed ID: 27077848]. [PubMed Central ID: PMC4849001]. https://doi.org/10.3390/ijms17040545.
-
7.
Sohn VY, Eckert MJ, Martin MJ, Arthurs ZM, Perry JR, Beekley A, et al. Efficacy of three topical hemostatic agents applied by medics in a lethal groin injury model. J Surg Res. 2009;154(2):258-61. [PubMed ID: 19329126]. https://doi.org/10.1016/j.jss.2008.07.040.
-
8.
Hajizadeh S, Shariati A, Jahani S, Haibar H, Haghighizadeh MH. Comparison of ChitoHem Powder and Sand Bag for Controlling Bleeding After Femoral Angiography. Jundishapur Journal of Chronic Disease Care. 2018;7(2).
-
9.
Arnaud F, Teranishi K, Tomori T, Carr W, McCarron R. Comparison of 10 hemostatic dressings in a groin puncture model in swine. J Vasc Surg. 2009;50(3):632-9, 639.e1. [PubMed ID: 19700097]. https://doi.org/10.1016/j.jvs.2009.06.010.
-
10.
Littlejohn LF, Devlin JJ, Kircher SS, Lueken R, Melia MR, Johnson AS. Comparison of Celox-A, ChitoFlex, WoundStat, and combat gauze hemostatic agents versus standard gauze dressing in control of hemorrhage in a swine model of penetrating trauma. Acad Emerg Med. 2011;18(4):340-50. [PubMed ID: 21496135]. https://doi.org/10.1111/j.1553-2712.2011.01036.x.
-
11.
Rembe JD, Bohm JK, Fromm-Dornieden C, Schafer N, Maegele M, Frohlich M, et al. Comparison of hemostatic dressings for superficial wounds using a new spectrophotometric coagulation assay. J Transl Med. 2015;13:375. [PubMed ID: 26620128]. [PubMed Central ID: PMC4666077]. https://doi.org/10.1186/s12967-015-0740-5.
-
12.
Monagle P, Barnes C, Ignjatovic V, Furmedge J, Newall F, Chan A, et al. Developmental haemostasis. Impact for clinical haemostasis laboratories. Thromb Haemost. 2006;95(2):362-72. [PubMed ID: 16493500]. https://doi.org/10.1160/th05-01-0047.
-
13.
Littlejohn L, Bennett BL, Drew B. Application of current hemorrhage control techniques for backcountry care: part two, hemostatic dressings and other adjuncts. Wilderness Environ Med. 2015;26(2):246-54. [PubMed ID: 25704877]. https://doi.org/10.1016/j.wem.2014.08.018.
-
14.
Ignjatovic V, Pelkmans L, Kelchtermans H, Al Dieri R, Hemker C, Kremers R, et al. Differences in the mechanism of blood clot formation and nanostructure in infants and children compared with adults. Thromb Res. 2015;136(6):1303-9. [PubMed ID: 26522267]. https://doi.org/10.1016/j.thromres.2015.10.034.
-
15.
Kozen BG, Kircher SJ, Henao J, Godinez FS, Johnson AS. An alternative hemostatic dressing: comparison of CELOX, HemCon, and QuikClot. Acad Emerg Med. 2008;15(1):74-81. [PubMed ID: 18211317]. https://doi.org/10.1111/j.1553-2712.2007.00009.x.
-
16.
Pozza M, Millner RW. Celox (chitosan) for haemostasis in massive traumatic bleeding: experience in Afghanistan. Eur J Emerg Med. 2011;18(1):31-3. [PubMed ID: 20461007]. https://doi.org/10.1097/MEJ.0b013e32833a5ee4.
-
17.
Sae-Jung S, Apiwatanakul P. Chitosan Pad, Cellulose Membrane, or Gelatin Sponge for Peridural Bleeding: An Efficacy Study on a Lumbar Laminectomized Rat Model. Asian spine journal. 2018;12(2):195.
-
18.
Monagle P, Barnes C, Ignjatovic V, Furmedge J, Newall F, Chan A, et al. Developmental haemostasis. Thrombosis and haemostasis. 2006;95(2):362-72.
-
19.
Chamanzari H, Choubdar M, Sharifipour F, Gholami H, Daneshvari F. Comparison of Celox powder and conventional dressing on hemostasis of vascular access site in hemodialysis patients. Evidence Based Care. 2015;4(4):25-34.
-
20.
Ramsay ES, Lerman MA. How to use the erythrocyte sedimentation rate in paediatrics. Arch Dis Child Educ Pract Ed. 2015;100(1):30-6. [PubMed ID: 25205237]. https://doi.org/10.1136/archdischild-2013-305349.

