Abstract
Background:
Depression is a mental health disorder, which affects all the communities around the world. One of the major components of depression is hopelessness. There is evidence that depression is accompanied by increased reactive oxygen species, which contribute to DNA damage.Objectives:
This study aimed at evaluating whether depression and hopelessness are associated with markers of DNA damage, proliferative potential, and cell death.Methods:
The sample included 59 healthy females from Fars province, southern Iran. Depression and hopelessness scores were measured using Beck’s depression inventory and Beck’s hopelessness scales, respectively. Smears were taken from the buccal mucosa of participants, and using micronucleus assay, they were analyzed for various cell population and genome damage markers, including cytokinetic defects (binucleated cells), proliferative potential (basal cell frequency), cell death (condensed chromatin, karyorrhectic, pyknotic, and karyolitic cells), and biomarkers of DNA damage (micronuclei and nuclear buds).Results:
None of the micronucleus (MN) assay parameters had an association with depression scores. There was a significant negative correlation between the frequency of basal cells and hopelessness scores (r = -0.323, P = 0.012). Other parameters of MN assay showed no association with hopelessness scores.Conclusions:
As the basal cells are biomarkers of proliferative potential, the results suggest that cellular regeneration is decreased in buccal mucosa of people with high level of hopelessness. However, more studies with larger sample sizes are required to verify this conclusion.Keywords
Basal Cells Cytological Parameters Depression Micronucleus Assay
1. Background
Depression is a very common mental health disorder, which affects all communities around the world. It is a complex disorder, which can be the result of interaction of environmental, genetic, and biological risk factors. Problems, which may be associated with depression, include sadness, hopelessness, feelings of guilt, worthlessness, helplessness, suicidal thoughts, and restlessness (1). Hopelessness is among symptoms strongly related to depression (2). Hopelessness is characterized by a feeling of futility and negative expectations of the future and one’s personal goals. Individuals, who are hopeless, lack anticipation of future positive events and they think their goals will not be achieved or they show conditional goal setting (3, 4).
Reactive oxygen species (ROS) and reactive nitrogen species (RNS), including radicals and other reactive oxygen/nitrogen species, such as superoxide, nitric oxide, and hydrogen peroxide, react with key cellular structures and alter their biological function. Under normal conditions, the production of ROS and RNS is tightly regulated and they have many physiological roles. However, in excess, ROS and RNS may react with fatty acids, proteins, and DNA, thereby, causing damage to these substrates (5). Furthermore, ROS is known to cause many different types of DNA lesions, including base modifications, DNA strand breaks, interest and cross links and micronuclei (6, 7). There is much evidence that suggests the involvement of ROS and RNS in the pathophysiology of depression and possibly its association with neurodegeneration. These findings show decreased antioxidant status, increases in ROS and RNS, and signs of damage caused by oxidative and nitrosative stress to fatty acids and DNA in patients with depression (5).
Oral exfoliative cytology is a microscopic examination and measurement of cells, which have been shed or removed from the buccal epithelial surface. The smear obtained by oral exfoliative cytology can be analyzed for buccal micronucleus assay (MN assay). Based on cytological and nuclear features, the MN assay has been used to measure genome damage, proliferative potential, and cell death (8). Biomarkers of this assay have been reported to be associated with neurodegenerative diseases (9). Considering the fact that impaired antioxidant defense and neurodegeneration are hallmarks of depression (5), the present study was carried out to assess whether depression and hopelessness are associated with parameters of MN assay.
2. Objectives
This study was carried out to evaluate whether depression and hopelessness are associated with markers of DNA damage, proliferative potential, and cell death.
3. Materials and Methods
3.1. Study subjects
The study was carried out on 59 healthy females, aged between 19 and 24 years old (mean ± SD: 20.75 ± 1.32). Sampling started on February 2015 and was completed by June 2016. All participants were from the Fars province, southern Iran. Exclusion criteria for the subjects included presence of diseases, alcohol or smoking habit, carrying restorative dental fillings, using any mouthwash, receiving any drug, dental treatment or recent facial or oral radiographs. This study was approved by the Shiraz University ethics committee (ECBDE-SU-9-6177616), and all subjects were informed about the objectives of the study.
3.2. Measurement of Hopelessness and Depressive Symptoms
Depression was measured with Beck’s depression inventory (BDI). Beck’s depression inventory is a 21-item self-report scale measuring participants’ levels of depression over the past week. The total scores range from 0 to 63, with higher scores indicating greater levels of depressive symptoms. The participants can be categorized to 4 groups, according to the following score ranges: 0 to 9, no depression; 10 to 18, low depression; 19 to 29, moderate depression; and 30 to 63, severe depression (10). The level of hopelessness was assessed using Beck’s hopelessness scale (BHS), a 20-item self-report scale designed to measure participants’ levels of hopelessness over the past week. The total scores ranged from 0 to 20, with higher scores indicating greater levels of hopelessness. For categorical levels of hopelessness, the score ranges were as follows: 0 to 3, no hopelessness at all; 4 to 8, mild hopelessness; 9 to 14, moderate hopelessness; and 15 to 20, severe hopelessness (11).
3.3. Cell Sampling and Preparation
Buccal cells (BCs) were collected from volunteers. Prior to BC collection, the mouth was rinsed thoroughly with water to remove any unwanted debris. Small-headed toothbrushes were rotated 30 times in a circular motion against the inside of the left cheek. The heads of the brush was placed in a 14-mL falcon (Maxwell) containing BC buffer (0.01 M Tris-HCl, 0.13 M EDTA, 0.02 M sodium chloride) at pH 7.0, and agitated to dislodge the cells. The cells were centrifuged (Iran Khodsaz) for 10 minutes at 500 g. The supernatant was removed and replaced with 10 mL of fresh BC buffer. Cells were spun and washed twice more. The supernatant was removed and cells were re-suspended in 3 ml of fixative (ethanol-acetic acid (3:1)). After 5 minutes, the cells were centrifuged, re-suspended in 200 microliter of fixative and dropped onto slides, and then allowed to air-dry for 10 minutes. Slides were treated with 5 M HCl for 30 minutes and were then stained with Schiff’s reagent for 60 minutes. The cells were counterstained with 0.2% light green for 30 seconds. Reagents were purchased from the Merck company (Germany) and BDH Chemicals Ltd (England).
3.4. Microscopic Observation
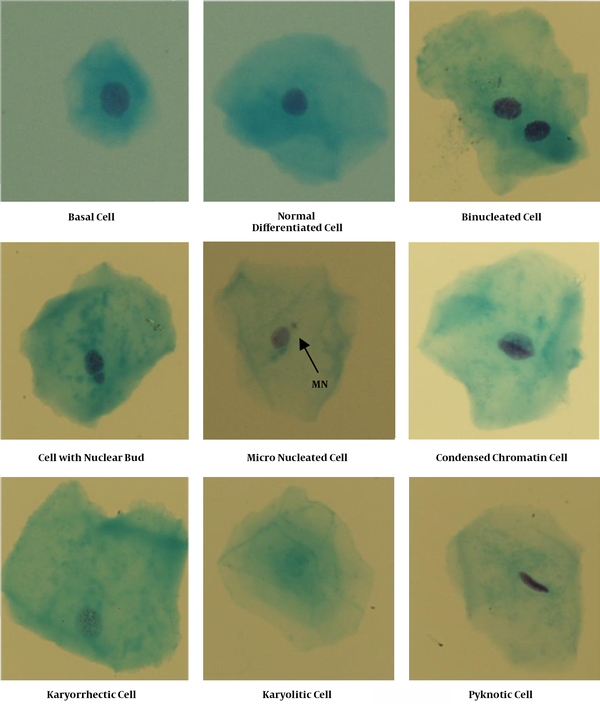
Using the MN assay, biomarkers of DNA damage (micronuclei and nuclear buds), cytokinetic defects (bi-nucleated cells), proliferative potential (basal cell frequency), and cell death (condensed chromatin, karyorrhectic, pyknotic, and karyolitic cells) could be evaluated (8). Various distinct cell populations were scored in MN assay (Figure 1) and identified based on the criteria outlined by Thomas et al. (12). According to these criteria, Basal cells have a uniformly stained nucleus. They have smaller size and larger nuclear-cytoplasmic ratio compared to differentiated cells. Normal differentiated cells have a uniformly stained nucleus. They have larger size and smaller nuclear-cytoplasmic ratio relative to basal cells. The micronucleated cells contain both the main nucleus and one or more smaller nuclei called Micronuclei (MNs). The MNs are round or oval with similar stain intensity as the main nucleus and have a diameter of 1/16 to 1/3 of the main nucleus. The cells with nuclear bud have nuclei attached to the main nucleus, suggestive of a budding process. The nuclear bud has a similar morphology and staining properties as the nucleus and its diameter may range from a quarter to half of the nucleus. Binucleated cells have 2 nuclei with the same morphology. The nuclei are in close proximity or may be touching each other. Condensed chromatin cells have a nucleus with striated pattern of parallel tracts of aggregated chromatin. The nucleus is intensively stained in distinct areas of chromatin condensation. The karyorrhectic cells have a nucleus with extensive chromatin aggregation, leading to fragmentation and disintegration of the nucleus. The pyknotic cells have a small and shrunken nucleus, which is uniformly and highly stained. The nuclear diameter is approximately 1/3 to 2/3rd of the normal nucleus. The karyolytic cells are cells with a DNA depleted nucleus. The nucleus has no Feulgen staining and appears as a ghost-like image. The frequency of each cell type was determined in 1000 cells for each person. The cells were analyzed under a total magnification of 400 × using a Nikon microscope.
Images Showing Distinct Buccal Cell Types as Scored in the Buccal Cytome Assay
3.5. Statistical Analysis
The normality of the variables was evaluated using the Shapiro-Wilk test. Since depression and hopelessness were distributed non-normally, Spearman’s correlation test was applied to determine the correlation between depression, hopelessness, and biomarkers evaluated by the MN assay. Also, to confirm that age made no difference, the correlation between age and either of depression or hopelessness was examined in the same way. Significance was accepted at P < 0.05. Statistical analysis was performed using the SPSS version 22 software.
4. Results
There was no significant correlation between age and either depression or hopelessness scores (r = 0.130, P = 0.326, r = 0.027, and P = 0.840, respectively). The mean of depression and hopelessness scores in the study subjects were 12.66 ± 9.29 (range of 0 to 38) and 4.93 ± 3.85 (range of 0 to 16), respectively. The distribution of depression and hopelessness scores, and also biomarkers of MN assay in the study subjects are displayed in Table 1. To determine the correlation between depression, hopelessness, and biomarkers of MN assay, including biomarkers of DNA damage, cytokinetic defects, proliferative potential and cell death, the Spearman’s correlation test was performed (Table 2). As expected, depression scores were significantly correlated with hopelessness scores. On the other hand, greater depression scores were associated with increased scores of hopelessness. None of the MN assay parameters had an association with depression scores. Although showing no significant correlation, it should be noticed that the mean of condensed chromatin and nuclear bud increased in individuals with high scores of depression (Table 1). However, in the case of hopelessness, there was a significant negative correlation between the frequency of basal cells and hopelessness scores. This negative correlation was also observed with depression, although it did not achieve significance. Other parameters of the MN assay showed no association with hopelessness scores.
Distribution of Depression and Hopelessness Scores and Biomarkers of Micronuclei Assay in the Study Subjectsa
| Variables | Sub, No. | Basal Cell | BN Cell | CC Cell | KH Cell | PK Cell | KL Cell | MN | NBUD |
|---|---|---|---|---|---|---|---|---|---|
| DS range | |||||||||
| 0 - 9 | 24 | 27.92 ± 11.92 | 6.38 ± 3.33 | 59.29 ± 30.82 | 93.25 ± 55.55 | 4.25 ± 4.63 | 83.92 ± 67.20 | .04 ± .20 | 12.96 ± 4.13 |
| 10 - 18 | 21 | 28.76 ± 13.64 | 6.81 ± 3.98 | 62.38 ± 30.01 | 96.52 ± 44.62 | 5.24 ± 7.06 | 91.76 ± 65.40 | .62 ± 1.91 | 13.14 ± 6.90 |
| 19 - 29 | 10 | 25.20 ± 9.94 | 5.80 ± 2.97 | 78.90 ± 42.89 | 83.20 ± 41.59 | 5.00 ± 4.19 | 115.90 ± 105.83 | .50 ± .97 | 14.10 ± 6.48 |
| 30 - 63 | 4 | 19.00 ± 6.37 | 6.00 ± .81 | 91.50 ± 56.28 | 56.75 ± 20.37 | 5.50 ± 6.45 | 103.00 ± 53.80 | .00 ± .00 | 16.50 ± 13.91 |
| HS range | |||||||||
| 0 - 3 | 26 | 30.62 ± 14.02 | 6.12 ± 3.63 | 61.62 ± 32.36 | 81.50 ± 47.40 | 5.65 ± 6.75 | 84.38 ± 70.23 | .54 ± 1.72 | 13.27 ± 5.53 |
| 4 - 8 | 20 | 27.20 ± 9.33 | 6.25 ± 3.21 | 67.60 ± 33.55 | 108.70 ± 52.45 | 4.55 ± 4.29 | 101.15 ± 86.65 | .05 ± .22 | 12.75 ± 5.98 |
| 9 - 14 | 11 | 21.36 ± 8.68 | 7.55 ± 3.41 | 61.73 ± 33.95 | 86.18 ± 33.43 | 2.27 ± 3.40 | 101.36 ± 55.41 | .36 ± .92 | 13.27 ± 5.83 |
| 15 - 20 | 2 | 13.50 ± .70 | 5.50 ± .70 | 127.50 ± 64.34 | 41.50 ± 16.26 | 10.50 ± 4.95 | 90.00 ± 76.36 | .00 ± .00 | 24.00 ± 18.38 |
| Total | 59 | 27.15 ± 12.01 | 6.41 ± 3.37 | 65.90 ± 35.12 | 90.24 ± 48.00 | 4.81 ± 5.55 | 93.42 ± 72.70 | .32 ± 1.22 | 13.46 ± 6.37 |
The Correlation Between Depression, Hopelessness and Biomarkers of the Micronuclei Assay
5. Discussion
Depression is a potentially life-threatening disorder, which can occur at any age and affects hundreds of millions of people all over the world (1). One of the main features of depression is hopelessness. In agreement with other studies, the result of this study indicates a significant positive correlation between depression and hopelessness scores (13, 14).
The MN assay has been shown to be an effective tool in measuring cytogenetic damage, which can be the result of lifestyle characteristics, occupational exposure, diseases, and environmental risk (8). The frequency of MN has been revealed to be increased in patients affected by neurodegenerative disorders, such as Alzheimer’s and Parkinson’s disease, and psychiatric disorders, such as anorexia nervosa and bulimia nervosa (9, 15). It has been shown that the number of MN in females with fibromyalgia was significantly higher than that of controls (16). Fibromyalgia is a multi-factorial disease and psychiatric disorders seem to play a crucial role in its pathophysiology (17).
According to the result of the present study, there was no correlation between depression score and biomarkers of the MN assay. However, in case of hopelessness, as the score increased, the frequency of basal cells significantly decreased. The oral epithelium is composed of 4 distinct layers, including connective tissue, basal cell layer containing actively dividing basal cells, prickle cell layer containing populations of differentiated, apoptotic and necrotic cells, and the keratinized cell layer, which lines the surface of oral cavity containing cells that are constantly being shed. The structure and integrity of the oral epithelium is maintained by progeny produced in the basal layer by mitosis migration to the surface replacing those that are shed (18). As the basal cells are biomarkers of proliferative potential, the results suggest that cellular regeneration is decreased in buccal mucosa of people with high level of hopelessness. Individuals with Alzheimer’s disease have also been revealed to have lower frequency of basal cells compared to controls (19); this finding, along with the results of the present study, may support a previous report, which suggested that hopelessness during midlife may increase the risk of cognitive impairment and Alzheimer’s disease in later life (20).
It should be noted that, although hopelessness and depression are significantly correlated, they did not have the same effect on the BCs profile. The frequency of basal cells decreased as the depression score increased, however, this association did not achieve significance. This finding indicates that hopelessness seems to have a distinct or stronger effect on cell renewal in buccal mucosa. The relative strength of the associations found for hopelessness versus depression has also been found by several other studies examining cardiovascular disease, hypertension, and suicide (13, 14, 21, 22). Although hopelessness is strongly related to depression, it is not necessary to cause depression; in fact, individuals may experience hopelessness without depression (23). The result of this study supports the notion that hopelessness may be distinct from depression.
5.1. Conclusion
This study was the first to examine the association of depression and hopelessness scores with biomarkers of the buccal micronucleus assay. Results showed a significant decrease in the frequency of basal cells in individuals with high score of hopelessness, suggesting reduced proliferative potential of the buccal mucosa in these people. If this preliminary finding is verified in larger studies and combined with further investigation of the possible effect of hopelessness on dementia, it could provide a basis to improve understanding of the biological mechanisms leading to the development of depressive and cognitive disorders.
5.2. Limitations
One of the main limitations of this study was small sample size which was the result of restricted exclusion criteria, especially dental filling. Since genotoxic damage increases in the oral mucosa cells of subjects with dental fillings (24), only the subjects with no dental fillings were included in the study. However, most people, even at young ages, have dental filling at least for one tooth.
Acknowledgements
References
-
1.
Khan M, Aqeel N, Abbas A. An overview of depression and its pharmacotherapy. Int J Res Pharmacology Pharmacotherapy. 2014;3(1):1-6.
-
2.
Beck AT. Depression: Clinical, experimental and theoretical aspects. New York: Harper and Row; 1967.
-
3.
Hadley SA, MacLeod AK. Conditional goal-setting, personal goals and hopelessness about the future. Cogn Emot. 2010;24(7):1191-8. https://doi.org/10.1080/02699930903122521.
-
4.
MacLeod AK, Rose GS, Williams JMG. Components of hopelessness about the future in parasuicide. Cognit Ther Res. 1993;17(5):441-55. https://doi.org/10.1007/bf01173056.
-
5.
Maes M, Galecki P, Chang YS, Berk M. A review on the oxidative and nitrosative stress (O&NS) pathways in major depression and their possible contribution to the (neuro)degenerative processes in that illness. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(3):676-92. [PubMed ID: 20471444]. https://doi.org/10.1016/j.pnpbp.2010.05.004.
-
6.
Cadet J, Berger M, Douki T, Ravanat JL. Oxidative damage to DNA: formation, measurement, and biological significance. Rev Physiol Biochem Pharmacol. 1997;131:1-87. [PubMed ID: 9204689]. https://doi.org/10.1007/3-540-61992-5_5.
-
7.
Xu B, Wang W, Guo H, Sun Z, Wei Z, Zhang X, et al. Oxidative stress preferentially induces a subtype of micronuclei and mediates the genomic instability caused by p53 dysfunction. Mutat Res. 2014;770:1-8. [PubMed ID: 25302047]. https://doi.org/10.1016/j.mrfmmm.2014.08.004.
-
8.
Yadav AS. Buccal micronucleus cytome assay- a biomarker of genotoxicity. J Mol Biomark Diagn. 2015;6(3). https://doi.org/10.4172/2155-9929.1000236.
-
9.
Migliore L, Coppede F, Fenech M, Thomas P. Association of micronucleus frequency with neurodegenerative diseases. Mutagenesis. 2011;26(1):85-92. [PubMed ID: 21164187]. https://doi.org/10.1093/mutage/geq067.
-
10.
Beck AT, Beck R, Kovacs M. Classification of suicidal behaviors: I. Quantifying intent and medical lethality. Am J Psychiatry. 1975;132(3):285-7. [PubMed ID: 1115273]. https://doi.org/10.1176/ajp.132.3.285.
-
11.
Beck AT, Weissman A, Lester D, Trexler L. The measurement of pessimism: the hopelessness scale. J Consult Clin Psychol. 1974;42(6):861-5. [PubMed ID: 4436473]. https://doi.org/10.1037/h0037562.
-
12.
Thomas P, Holland N, Bolognesi C, Kirsch-Volders M, Bonassi S, Zeiger E, et al. Buccal micronucleus cytome assay. Nat Protoc. 2009;4(6):825-37. [PubMed ID: 19444240]. https://doi.org/10.1038/nprot.2009.53.
-
13.
Whipple MO, Lewis TT, Sutton-Tyrrell K, Matthews KA, Barinas-Mitchell E, Powell LH, et al. Hopelessness, depressive symptoms, and carotid atherosclerosis in women: the Study of Women's Health Across the Nation (SWAN) heart study. Stroke. 2009;40(10):3166-72. [PubMed ID: 19713542]. https://doi.org/10.1161/STROKEAHA.109.554519.
-
14.
Everson SA, Kaplan GA, Goldberg DE, Salonen JT. Hypertension incidence is predicted by high levels of hopelessness in Finnish men. Hypertension. 2000;35(2):561-7. [PubMed ID: 10679498]. https://doi.org/10.1161/01.HYP.35.2.561.
-
15.
Torres-Bugarin O, Pacheco-Gutierrez AG, Vazquez-Valls E, Ramos-Ibarra ML, Torres-Mendoza BM. Micronuclei and nuclear abnormalities in buccal mucosa cells in patients with anorexia and bulimia nervosa. Mutagenesis. 2014;29(6):427-31. [PubMed ID: 25232046]. https://doi.org/10.1093/mutage/geu044.
-
16.
Menzies V, Lyon DE, Archer KJ, Zhou Q, Brumelle J, Jones KH, et al. Epigenetic alterations and an increased frequency of micronuclei in women with fibromyalgia. Nurs Res Pract. 2013;2013:795784. [PubMed ID: 24058735]. https://doi.org/10.1155/2013/795784.
-
17.
Ostilio P, Vincenzo C, Fabrizio P, Antonella M, Alfonso M, Daniela S. Psychiatric disorders, stress reaction and fibromyalgia syndrome (FMS): Report of an experience on 48 Patients. Ann Depress Anxiety. 2014;1(3):1012.
-
18.
Holland N, Bolognesi C, Kirsch-Volders M, Bonassi S, Zeiger E, Knasmueller S, et al. The micronucleus assay in human buccal cells as a tool for biomonitoring DNA damage: the HUMN project perspective on current status and knowledge gaps. Mutat Res. 2008;659(1-2):93-108. [PubMed ID: 18514568]. https://doi.org/10.1016/j.mrrev.2008.03.007.
-
19.
Thomas P, Hecker J, Faunt J, Fenech M. Buccal micronucleus cytome biomarkers may be associated with Alzheimer's disease. Mutagenesis. 2007;22(6):371-9. [PubMed ID: 17709794]. https://doi.org/10.1093/mutage/gem029.
-
20.
Hakansson K, Soininen H, Winblad B, Kivipelto M. Feelings of Hopelessness in Midlife and Cognitive Health in Later Life: A Prospective Population-Based Cohort Study. PLoS One. 2015;10(10). e0140261. [PubMed ID: 26460971]. https://doi.org/10.1371/journal.pone.0140261.
-
21.
Everson SA, Goldberg DE, Kaplan GA, Cohen RD, Pukkala E, Tuomilehto J, et al. Hopelessness and risk of mortality and incidence of myocardial infarction and cancer. Psychosom Med. 1996;58(2):113-21. [PubMed ID: 8849626]. https://doi.org/10.1097/00006842-199603000-00003.
-
22.
Beck AT, Steer RA, Kovacs M, Garrison B. Hopelessness and eventual suicide: a 10-year prospective study of patients hospitalized with suicidal ideation. Am J Psychiatry. 1985;142(5):559-63. [PubMed ID: 3985195]. https://doi.org/10.1176/ajp.142.5.559.
-
23.
Greene SM. The relationship between depression and hopelessness. Implications for current theories of depression. Br J Psychiatry. 1989;154:650-9. [PubMed ID: 2597858]. https://doi.org/10.1192/bjp.154.5.650.
-
24.
Visalli G, Baluce B, La Maestra S, Micale RT, Cingano L, De Flora S, et al. Genotoxic damage in the oral mucosa cells of subjects carrying restorative dental fillings. Arch Toxicol. 2013;87(1):179-87. [PubMed ID: 22872142]. https://doi.org/10.1007/s00204-012-0915-2.