Abstract
Introduction:
Angioedema is a serious reaction characterized by edema of the deep dermal and subcutaneous tissues.Case Presentation:
A 38-year-old woman was admitted to Ibn-e-Sina psychiatric hospital (Mashhad, Iran) in 2010, with amphetamine-induced mood disorder with onset during intoxication. She began taking sodium valproate, and later, risperidone treatment was started. On the 9th day of risperidone treatment, drug-induced angioedema occurred. Laboratory tests showed an elevated erythrocyte sedimentation rate and elevated C-reactive protein, with normal C3, C4, and C1 esterase inhibitor concentrations.Conclusions:
The risperidone treatment was discontinued, and only hydroxyzine and a low dose of hydrocortisone were prescribed. The edema resolved completely within 4 days.Keywords
1. Introduction
Atypical antipsychotics play a potent role in the treatment of psychotic disorders; however, they are accompanied by adverse cutaneous reactions in approximately 5% of users. The majority of these reactions, such as urticaria, are benign and easily managed, but life-threatening side effects such as angioedema are clinically more important (1). Angioedema is characterized by edema of the deep dermal and subcutaneous tissues. Swelling may be pale or red, with or without pruritus. Angioedema usually occurs in the face, lips, tongue, hands, or feet, and it can suffocate the patient if the swelling affects the tongue or upper airways. Drug-induced angioedema may be the result of three mechanisms: allergic reaction, a consequence of C1 esterase inhibitor deficiency, or aggregation of bradykinin. All of these mechanisms cause edema through increased capillary leakage due to inflammatory mediators (1). C1 esterase inhibitor may be suppressed by risperidone, causing edema to appear (2). This report presents a case of angioedema in a patient receiving risperidone.
2. Case Presentation
An unmarried 38-year-old woman was admitted to Ibn-e-Sina psychiatric hospital in Mashhad, northeastern Iran, in 2010. Her education level was a diploma and she was unemployed. She was aggressive and irritable, and had logorrhea and grandiose thoughts. Her symptoms had begun gradually two weeks before admission. This scenario continued despite psychiatric consultation and drug prescription.
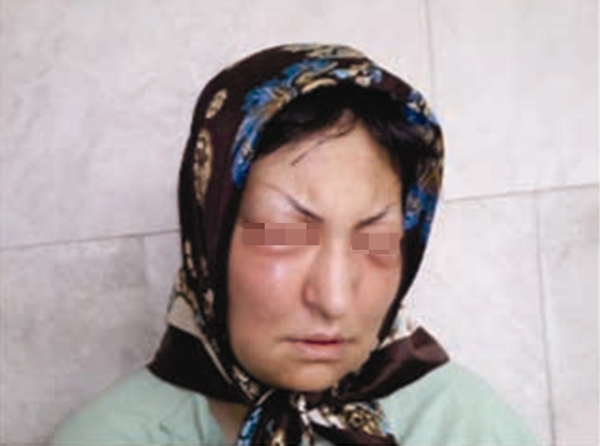
The patient had been diagnosed with polysubstance dependence, and at the time of admission, she was amphetamine-dependent (using a substance called “shishe” in Iran). She was alert and oriented. All laboratory test results and physical and neurological examinations were in the normal range. The patient was diagnosed with amphetamine-induced mood disorder with onset during intoxication. The patient began taking sodium valproate at an initial dose of 600 mg/day, which was then increased to 1,200 mg/day. Later, risperidone treatment was started with an initial dose of 2 mg/day, then increased to 6 mg/day. On the 9th day of risperidone treatment, facial and periorbital edema occurred suddenly (Figure 1). However, her vital signs were normal. Laboratory tests showed elevated erythrocyte sedimentation rate and C-reactive protein. She underwent a dermatology consultation, and the diagnosis was drug-induced angioedema. Evaluation of the complement system showed a normal C3 concentration of 130 mg/dL (reference range: 84 - 184 mg/dL) and a normal C4 concentration of 30 mg/dL (reference range: 25 - 70 mg/dL). C1 esterase inhibitor concentration was at a normal level of 51 mg (reference range: 8 - 40 mg/dL).
Facial and periorbital edema on the 9th day of risperidone treatment in a 38-year-old woman (risperidone-induced angioedema). The patient’s written permission to use her picture is available.
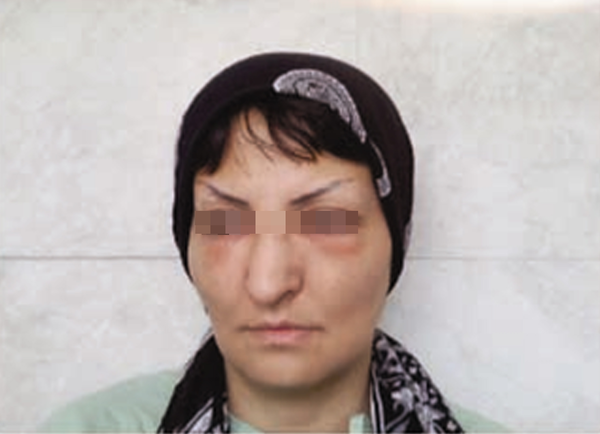
We decided to discontinue the risperidone treatment, and only hydroxyzine and a low dose of hydrocortisone were prescribed. The edema resolved completely within 4 days (Figure 2). The patient was temporarily discharged from the hospital at her family’s firm request, and she has not been readmitted. We received informed consent from the patient to publish about this side effect of risperidone during her hospitalization.
Complete resolution of edema on the 4th day after discontinuation of risperidone. The patient’s written permission to use her picture is available.
3. Discussion
Our patient’s immunological investigations were normal, indicating an acquired form of angioedema. There are a few international case reports about this rare side effect (3-5). In a case reported by Cooney in 1995, immunological investigations showed a low concentration level of C1 esterase inhibitor. That case was a hereditary type of angioedema, as the concentrations of complement components and the plasma levels of C1 esterase inhibitor were low (3).
Kores Plesnicar et al. described the side effect of angioedema in a 63-year-old female who developed it on three occasions when exposed to risperidone. Each time, the edema subsided with discontinuation of the drug. Due to a low level of C4, low levels of markers of classical complement-activation pathways, and normal IgE levels, the author introduced it as an allergic reaction (4). Erken et al. (2) reported a 34-year-old female with postpartum psychosis, who had a history of valproic acid use for 2 years for the treatment of epilepsy. Risperidone was begun for her at a dose of 4 mg/day, then it was increased to 6 mg/day. Angioedema appeared in the 2nd week after the initiation of risperidone (5). In 2010, Soumya reported angioneurotic edema with risperidone in a 15-year-old boy with schizophrenia (6). In that case, risperidone was started at 1 mg/d and increased to 2 mg/d after 2 weeks. The patient developed edema over his face and feet. The facial edema was not accompanied by other symptoms, such as fever, lymphadenopathy, or dyspnea. Routine laboratory tests were within normal ranges; however, complement levels were not measured. Risperidone was discontinued and the edema subsided within one week (6). In 2013, Gunes et al. reported periorbital edema, dyspnea, and dysphagia induced by risperidone in a schizophrenic woman, 3 days after a 25-mg muscular injection of the drug. Her physical exam and laboratory tests were normal. This complication resolved after risperidone discontinuation (7).
In previous studies about angioedema as a side effect of risperidone, all of the angioedema cases occurred during the 1st month of taking the drug, as in the present case. There is no report of angioedema occurring more than 30 days after the prescription of risperidone. In addition, 39.13% of angioedema cases occur in females and 60.7% in males. The highest rate of angioedema manifests in the age ranges of 40 - 49 and > 60 years.
To treat this type of angioedema, the causative antipsychotic should be discontinued. In severe cases, maintaining a patent airway is critical. If any signs of a compromised airway are detected, adrenaline should be prescribed immediately. Steroids and antihistamines may also be useful in allergic reactions.
A review of the data suggests that this side effect of risperidone may be dose-dependent; therefore, we recommend that whenever possible, the subject should not be re-challenged, as the edema may recur. If there is no alternative, a lower dose may be safe. Considering the life-threatening nature of edema in severe cases, physicians should be aware of this important side effect and inform their patients about early symptoms. Cross-reactivity between antipsychotics has not been reported.
References
-
1.
Warnock JK, Morris DW. Adverse cutaneous reactions to antipsychotics. Am J Clin Dermatol. 2002;3(9):629-36. [PubMed ID: 12444805].
-
2.
Erken DD, Kilico O, Okay IT, Dilbaz N. A case of angioedema due to risperidone. Bull Clin Psychopharm. 2007;17(4):198-202.
-
3.
Cooney C, Nagy A. Angio-oedema associated with risperidone. BMJ. 1995;311(7014):1204. [PubMed ID: 7488898].
-
4.
Kores Plesnicar B, Vitorovic S, Zalar B, Tomori M. Three challenges and a rechallenge episode of angio-oedema occurring in treatment with risperidone. Eur Psychiatry. 2001;16(8):506-7. [PubMed ID: 11777744].
-
5.
Van Melick EJ. [Atypical antipsychotics in the elderly]. Tijdschrift voor Gerontologie en Geriatrie. 2004;35(6):240-5.
-
6.
Soumya RN, Grover S, Dutt A, Gaur N. Angioneurotic edema with risperidone: a case report and review of literature. Gen Hosp Psychiatry. 2010;32(6):646 e1-3. [PubMed ID: 21112458].
-
7.
Gunes F, Batgi H, Akbal A, Canatan T. Angioedema - an unusual serious side effect of risperidone injection. Clin Toxicol (Phila). 2013;51(2):122-3. [PubMed ID: 23336748].