Abstract
Background:
Silkworm products were first used by physicians more than 8500 years ago, in the early Neolithic period. In Persian medicine, silkworm extract has several uses for treating and preventing neurological, cardiac, and liver diseases. Mature silkworms (Bombyx mori) and their pupae contain a variety of growth factors and proteins that can be used in many repair processes, including nerve regeneration.Objectives:
The study aimed to evaluate the effects of mature silkworm (Bombyx mori), and silkworm pupae extract on Schwann cell proliferation and axon growth.Methods:
Silkworm (Bombyx mori) and silkworm pupae extracts were prepared. Then, the concentration and type of amino acids and proteins in the extracts were evaluated by Bradford assay, sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and liquid chromatograph-mass spectrometer (LC-MS/MS). Also, the regenerative potential of extracts for improving Schwann cell proliferation and axon growth was examined by 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide (MTT) assay, electron microscopy, and NeuroFilament-200 (NF-200) immunostaining.Results:
According to the results of the Bradford test, the total protein content of pupae extract was almost twice that of mature worm extract. Also, SDS-PAGE analysis revealed numerous proteins and growth factors, such as bombyrin and laminin, in extracts that are involved in the repair of the nervous system. In accordance with Bradford's results, the evaluation of extracts using LC-MS/MS revealed that the number of amino acids in pupae extract was higher than in mature silkworm extract. It was found that the proliferation of Schwann cells at a concentration of 0.25 mg/mL in both extracts was higher than the concentrations of 0.01 and 0.05 mg/mL. When using both extracts on dorsal root ganglion (DRGs), an increase in length and number was observed in axons.Conclusions:
The findings of this study demonstrated that extracts obtained from silkworms, especially pupae, can play an effective role in Schwann cell proliferation and axonal growth, which can be strong evidence for nerve regeneration, and, consequently, repairing peripheral nerve damage.Keywords
Silkworm (Bombyx mori) Extract Pupae Extract Dorsal Root Ganglia Schwann Cell Proliferation Axon Growth
1. Background
Peripheral nerve injuries are the most common nervous system injuries, affecting 20 million people in the United States and over 300,000 people in Europe each year (1-3). In 2 - 5% of all trauma cases, such as sports and vehicle accidents or iatrogenic damage, peripheral nerve injury occurs, while traffic and industrial accidents, natural disasters, and war damage are the most common causes of peripheral nerve injury (4-7). After the injury, mature Schwann cells (SCs) can transform into repair SCs, proliferate, promote the regeneration process, and finally differentiate again (8-10). Schwann cells support the regeneration of injured axons by secretion of a variety of neurotrophic factors, such as brain-derived neurotrophic factor (BDNF), glial cell-derived neurotrophic factor (GDNF), and cerebral dopamine neurotrophic factor (CDNF), which enhance the growth of regenerating axons after acute or chronic peripheral nerve injuries (11-13). Also, SCs arrange themselves longitudinally to form Büngner bands, which serve as axon regeneration channels (4, 14, 15). The peripheral nerve injuries also trigger a series of factors known as Wallerian degeneration, a process of retrograde degeneration of the distal end of an axon, which results in the production of fragments of both axon and myelin (16-18). After Wallerian degeneration, SCs, in association with macrophages, migrate to the injury site and participate in phagocytosis of myelin debris, in addition to creating myelin sheaths (8, 13, 19). However, based on clinical evidence, inherently slow axonal regeneration (less than 1 mm/day) and prolonged periods of denervation cause poor functional recovery after peripheral nerve injuries (20-23). Additionally, significant SC death occurs after cell transplantation, limiting its therapeutic efficacy (24-26). As a result, it is critical to improving the regenerative potential of SCs by providing either drugs or neurotrophic factors at the site of injury to enhance peripheral axon regeneration.
Some silkworm (Bombyx mori) products are the oldest natural substances known to have accompanied humanity throughout history. In the early Neolithic period, Silkworm products were first employed more than 8500 years ago (27). Silkworm pupae oil, chitin, and chitosan have several uses in the food industry, cosmetics, agriculture, biomedicine, textiles, biotechnology, and wound healing agents, etc. (28). Silk (so-called Abresham) has been used in Persian medicine by Persian physicians (also called Hokama), such as Rhazes (AD8th century) and Avicenna (AD10th century). Its applications included treating and preventing neurological, cardiac, and liver diseases. Moreover, Aghili Khorasani in the book, Makhzan-al-Adawiyah, in AD18th century (29) and Hakim Mohammad Momen in the book, Tahfato-al-Momenin in the AD17th century (30) specified that silkworm dressing (Osro or Yasroo Zamad) is viable in repairing severed nerves and blood vessels (31). The cocoon silk produced by the larvae of the domestic silk moth Bombyx mori, which is made up of two major proteins, fibroin, and sericin, was used in the first experiments. However, because sericin has been shown to elicit the inflammatory response in vivo, complete removal of sericin, also known as degumming, is required before it can be used (32). However, purified sericin has been shown to influence cartilaginous tissue regeneration, promoting cell regeneration, skin tissue regeneration, and the healing process with low levels of the inflammatory response (33). Due to its outstanding biocompatibility and tunable degradation qualities, silk fibroin is a promising biomaterial for tissue engineering and regenerative medicine. Also, silkworms and some parts of their cycle have many growth factors and proteins, such as glycoprotein, laminin, and Bombyrin. Neurons and SCs have been successfully cultured using silk fibroin and growth factors extracted from silkworms (34, 35). For example, Bombyrin has roles in brain development, wound healing in the brain, and the repair process of the brain, glycoprotein has roles in development and growth (36), apolipoprotein has roles in regenerating nerve tissue in damaged rat neurons and regenerating peripheral nerve of rats (36-38) and arylphoring storage protein in reproduction (36).
2. Objectives
In the present study, we tried to investigate the effect of mature silkworm extract and its pupae on SC proliferation and axonal growth based on the anecdotal and historical information available from the past to the present, especially within the area of the use of therapeutic efficacy of silkworm Bombyx mori (B.m) harvests (31). While preparing the extract of mature silkworm and pupae and determining the types of proteins and amino acids in them, and determining their amounts in both extracts, we determined the effective doses and, finally, the most effective dose in SC proliferation and axon growth. These doses were explored in this study for the first time.
3. Methods
3.1. Materials
Dulbecco′s Modified Eagle′s Medium (DMEM), fetal bovine serum (FBS), and bovine pituitary extract (BPE) were purchased from Invitrogen. Poly-D-lysin (PDL), 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide (MTT), penicillin/streptomycin and trypsin/EDTA 0.25% were supplied from Sigma-Aldrich. Adult Bombyx mori worms and pupae were provided by the Iran silk research center (Gilan province). Newborn Wistar rats were bred in an animal nest of the Pediatric Urology and Regenerative Medicine Research Center.
3.2. Preparation of Extracts from Mature Silkworm and Silkworm Pupae
The digestive tract and its contents were discarded from the mature silkworms. Then, 30 g of the remained tissue was homogenized with liquid nitrogen in a mortar according to the protocol described in the literature (39). The homogenized tissue was mixed with distilled water in a ratio of 1 to 4 (v/v, 1:4) and stirred for 20 minutes before being placed on ice for 40 minutes. The supernatant was centrifuged for 30 minutes at 15,000 g. Bradford assay was then performed to determine the protein content of the extract. The same method was used to prepare the silkworm pupae extract. Only the pupae do not need to be dissected at first to remove the digestive tract.
3.3. Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis Electrophoresis Analysis
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) electrophoresis analysis was performed according to the protocol described in the literature (40). First, the polyacrylamide gel, which is prepared from two parts of stacking gel and resolving gel, was placed in the electrophoresis tank, and the 1X tank buffer was poured into the upper chamber. Then, marker solution (Thermos Scientific, Cat no.26614 Page Ruler TM Unstained Protein Ladder), mature silkworm extract solution (containing 30 µg of extract, 14 µg of 2X sample buffer, and 2 µL of 1X sample buffer), and silkworm pupae extract solution (containing 30 µg of extract, 3.53 µL of 2X sample buffer and 22.94 µL of 1X sample buffer) were transferred to the wells in non-reduction condition, and the 1X tank buffer was poured into the lower chamber. Finally, the samples were separated under 100 volts for 75 minutes. After electrophoresis, the gels were stained (40) with the colloidal Coomassie blue and then scanned.
3.4. Liquid Chromatograph-Mass Spectrometer Analysis
The liquid chromatograph-mass spectrometer (LC-MS/MS) analysis was carried out in the following order based on the literature (39-41). Both samples of mature silkworm and silkworm pupae extracts were centrifuged three times at 4000 rpm for 15 minutes and filtered using a membrane sterile syringe filter of 0.22 µm. Then, 10 µL were taken from each extract and added to the internal standard solution (200 µL per well), vortexed for 10 minutes, and centrifuged at 10,000 rpm for 4 minutes at 4°C. The supernatant was transferred to the microplate (150 µL) and dried under a nitrogen stream at 45°C for 20 minutes. Afterward, 50 µL of the daily working hydrochloric acid-1-butanol solution was added to each well, shaken for a minute, and incubated for 45 minutes at 65°C. Then, 50 µL of the acetonitrile solution 75% was added per well and shaken for 2 minutes, and the plate was covered eventually, and 10 µL of the arranged supernatant was infused into LC-MS/MS for examination.
3.5. Schwann Cell Isolation and Expansion
Experiments and animal care were provided by Tehran University of Medical Sciences (TUMS) guidelines, and all animal protocols were reviewed and approved by the state animal ethical committee. The SCs were derived from sciatic nerves of the 2- to 3- day-old Wistar rats and purified according to the protocol reported in the literature with some modifications (42). In brief, the epineurium was excised from the sciatic nerves. The nerves were then separated into multiple explants, which were then placed in PDL-covered plates filled with DMEM supplemented with 10% FBS, 1% penicillin/streptomycin, 2 µgmL-1 BPE, and cultured at 37°C with 90% humidity and 5% CO2. Explants were sub-cultured three to four times for two weeks to limit the likelihood of fibroblast contamination. After that, the explants were digested for one hour at 37°C with 0.125% collagenase. Then, an equivalent volume of the trypsin-EDTA 0.25% was added. After another 15 minutes of incubation at 37°C, the nerve segments were physically dissociated until they formed a homogenous suspension. After centrifuging at 1000 rpm for 5 minutes at 4°C, cell pellets were collected. The cells were re-suspended in full culture media and cultured on 35 mm PDL-coated tissue culture plates. The amount of SC cultures was affirmed by S-100 immunostaining.
3.6. Schwann Cell Morphological Analysis (Scanning Electron Microscopy)
The effect of different extracts on the morphology of SCs was evaluated by scanning electron microscopy (SEM). The SCs were cultured on PDL-coated coverslips for 24 hours and exposed to different extracts. After five days of incubation, SCs were rinsed twice with PBS and fixed in 4% paraformaldehyde for 10 minutes. The cells were dehydrated in increasing concentrations of ethanol (30 - 100 %) before drying in the air. Then, the specimens were sputter-coated with a 7 nm coating of gold before morphological analysis using an SEM device (VEGA, TESCAN, Czech Republic) at 5000x and 30,000x magnifications.
3.7. Schwann Cell Proliferation Assay
The MTT was used for proliferation analysis. The SCs were detached with trypsin-EDTA 0.125% and seeded at a density of 104 cells per well in a final volume of 100 µL/well in 96-well cell culture plates. This study was performed in two steps. In the first step, viability was examined using MTT assay three days after cell seeding. This step was repeated two times to confirm the validity of the obtained results. Various concentrations of mature silkworm and silkworm pupae extracts, including 0.005, 0.01, 0.05, 0.25, 1.25, 5, and 25 µg/µL, were evaluated to obtain the effective concentration for improving SC proliferation. In the next step, SC proliferation was studied at 3, 5, and 7 days of exposure to the effective concentrations. The cell culture media was aspirated correctly at each time point, and the wells were filled with 100 µL of MTT solution (0.5 mg/mL in PBS). The plates were incubated for 3 hours at 37°C. The reaction solution was carefully removed from each well, and 100 µL of DMSO was added and shaken gently until the generated dark blue formazan crystals were dissolved. Using an enzyme-linked immunosorbent assay (ELISA) reader equipment, the absorbance was measured at wavelengths ranging from 405 to 630 nm (ElX-800, Bio-Tek Inc.).
3.8. Dorsal Root Ganglion Culture
Dorsal root ganglion (DRGs) are frequent models for peripheral nerve regeneration and were chosen for the research because of their significance in the field and ease of isolation and culture (43). The major sensory neurons responsible for transducing and modifying sensory information and transferring it to the spinal cord have their cell bodies in the DRG. Dorsal root ganglion neurons are divided into many categories based on the size of their cell bodies and their function (39). We stimulated the DRGs using different doses of mature silkworm and pupal extracts in the present study. Then, we investigated the effect of these extracts on the axon outgrowth from DRGs in terms of length and number using Image J software.
Dorsal root ganglion were harvested from 2-day-old Wistar rats and cultured as described previously (40, 41, 44, 45) with some modifications. Briefly, DRGs were removed and collected in ice-cold medium (DMEM with 1% penicillin-streptomycin) and then placed on PDL-coated 6-well plates (three DRGs per each well), filled with DMEM, 10% FBS, and 1% penicillin-streptomycin, and then were incubated at 37°C at a 90% humidified atmosphere with 5% CO2. After 48 h, the effective concentrations of adult silkworm and silkworm pupae extracts based on the MTT assay results (i.e., concentrations of 0.01, 0.05, 0.25 μg/μL) were added to the plates containing DRGs. Then, they were transferred to an incubator and monitored daily. After 12 days, DRGs were immune-stained for NeuroFilament-200 (NF-200).
3.9. Immunostaining Procedures for S-100 and Alpha-Smooth Muscle Actin
The SCs were seeded on coverslips. After 24 hours, the cells were fixed with 4% paraformaldehyde in 0.1 M PBS for 10 minutes at room temperature. The cells were then permeabilized for 30 minutes in PBS with 0.3% triton X-100 and washed with PBS three times. Endogenous peroxidase activity was blocked by 3% H2O2 (hydrogen peroxide) in 50% methanol for 10 min at room temperature. Cells were then blocked using 10% horse serum (Vector Laboratories) and then incubated overnight with primary antibodies, including mouse anti-SMA, MAD-021195Q, 1:100 dilution and anit-S-100 antibody, MAD-001221Q, 1:100 dilutions, master diagnostic, at 4°C. The next day, three PBS washes were followed by incubation for one hour at room temperature in secondary antibody (MAD- 000237QK, master diagnostic) biotinylated horse-anti-mouse IgG (1:200; Vector Laboratories), and colorimetric detection was performed using ABC Vectastain Elite reagents with DAB (Vector Laboratories). Sections were counterstained with hematoxylin (Sigma-Aldrich). Images of stained cells were captured using an Olympus BX-51 microscope.
3.10. Immunofluorescent Staining for NF200
First, the cells inside the plate were fixed for 10 minutes at room temperature with 4% paraformaldehyde in 0.1 M PBS. The cells were then permeabilized for 30 minutes in PBS with 0.3% triton X-100 and washed with PBS three times. They were then immersed in the blocking solution (10% goat serum) for 45 minutes and treated with primary antibody (anti-NF200, sc-32729-santa Cruz antibody) overnight at 4°C. The cells were then treated for one hour in a dark place with a FITC-conjugated secondary antibody (Goat Anti-Mouse IgG (H+L) antibody, 1:400 dilution). Also, DAPI (D9542-Sigma) was applied to counterstain the nuclei. Only the secondary antibody was employed as a negative control. Fluorescent imaging (with an Olympus microscope) was then performed.
3.11. Statistical Analysis
The independent student t-test or one-way analysis of variance (ANOVA) was used to assess the results. The mean ± standard error (SE) from at least three consecutive experiments is presented. If P-values < 0.05 or less, a statistically significant difference is reported.
4. Results
4.1. Mature Bombyx mori and Bombyx mori Pupae Extract Analysis
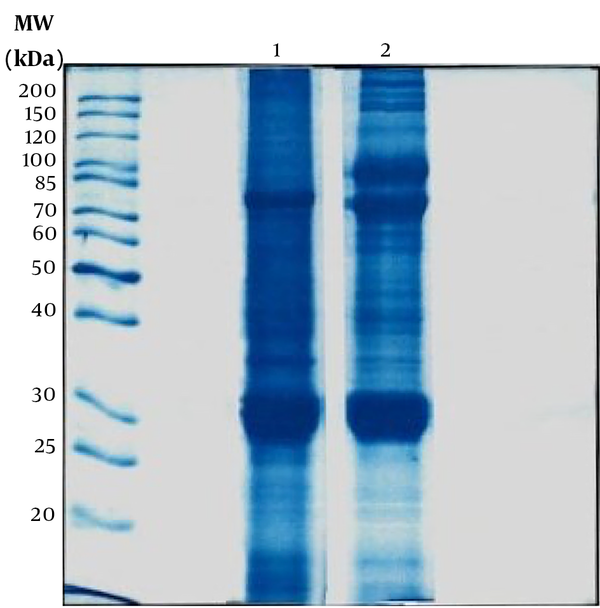
The Bradford test was performed to determine the total protein content of the prepared extracts. The average protein content determined from four samplings for mature silkworm extract was 2.412 mg/mL, and silkworm pupae extract was 5.078 mg/mL. Also, one-dimensional electrophoresis (SDS-PAGE) was performed to determine the protein bands of both extracts (Figure 1). The molecular weights of the proteins of the extracts appeared in the range of 20 - 200 kDa, and based on the literature, these bands can be attributed to the following proteins: fibroin (light & Heavy chains and P25), sericin, glycoprotein, lipoprotein, lipophorin, ferritin (light & heavy chains), apolipophorin-ІІІ, bombyrin, laminin511-E8 (α5, β1, γ1), and arylphorin storage protein (SP1 & SP2). Also, the optical density of proteins was calculated with Quantity One software and listed in Table 1. In addition, the type and number of amino acids in both extracts are summarized and compared in Table 2 and Figure 2.
One-dimensional electrophoresis sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Column 1 is related to mature silkworm extract, and column 2 is related to silk pupae extract. The molecular weights of the proteins of the extracts appeared in the range of 20 - 200 kDa.
The List of Proteins Detected in Mature Silkworm and Pupae Extract with Their Molecular Weight and Optical Density
| Row | Protein | Molecular Weight (kDs) | Optical Density Silkworm Extract | Relative Quality of Silkworm Extract | The Optical Density of Pupae Extract | The Relative Quality of Pupae Extract | Role of Protein | References |
|---|---|---|---|---|---|---|---|---|
| 1 | Fibroin consisting of a heavy chain, a light chain, and a glycoprotein, P25 | 26, 130, 180, 30 | 0.98, 0.36, 0.42, 1.05 | 9.7, 1.5, 2.6, 9.4 | 0.82, 0.44, 0.36, 0.80 | 9.4, 1.2, 1.7, 9.6 | Regenerative medicine, tissue engineering | Dai et al. (46); Dong et al. (47); Yonesi et al. (27) |
| 2 | Sericin | 150 | 0 | 0 | 0.36 | 1.7 | Regenerative medicine, tissue engineering | Dai et al. (46); Dong et al. (47) |
| 3 | Glycoprotein | 27 | 1.43 | 7.5 | 1.37 | 8.6 | Development | Chandrasekar and Habeeb (48); Maenaka and Park (36) |
| 4 | Lipoprotein, Lipophorin | 26, 29, 30, 31 | 0.98, 1.05, 1.05, 0.49 | 9.7, 9.4, 9.4, 2.2 | 0.82, 0.80, 0.80, 0.26 | 9.4, 9.6, 9.6, 1.0 | Transport of sterols and hormones, immunity, reproduction, prolonging life, and inhibiting programmed cell death | Chandrasekar and Habeeb (48); Maenaka and Park (36) |
| 5 | Ferritin (L-type), (H-type) | 19, 21 | 0, 0.19 | 0, 0.8 | 0, 0.10 | 0, 0.7 | Growth, differentiation, oxygen transport, storage, energy production, cell proliferation, cell cycle, RNA/DNA synthesis, immune response, anti-oxidation, iron homeostasis, detoxification, development regulation, and immunity | Chandrasekar and Habeeb (48); Maenaka and Park (36) |
| 6 | Apolipophorin-III | 22 | 0.24 | 1.4 | 0.11 | 0.7 | Facilitate lipid transport and immune responses against various pathogens | Chandrasekar and Habeeb (48); Maenaka and Park (36) |
| 7 | Bombyrin | 27 | 1.43 | 7.5 | 1.37 | 8.6 | Making contact with different molecules throughout the protein surface, the repair process of the brain | Chandrasekar and Habeeb (48); Maenaka and Park (36) |
| 8 | Laminin511-E8-α5, Laminin511-E8-β1, Laminin511-E8-γ1 | 90, 25, 30 | 0.42, 1.43, 1.05 | 1.5, 7.5, 9.4 | 0.73, 1.37, 0.80 | 16.4, 8.6, 9.6 | Extracellular protein in the basement membranes, regenerative medicine, human disease modeling, and drug discovery | Asakura et al. (49); Maenaka and Park (36); Tomita (50); Yonesi et al. (27) |
| 9 | Arylphorin storage protein (SP1) & (SP2) | 82, 72, 76 | 0.42, 0.81, 0.42 | 1.9, 5.0, 1.9 | 0.73, 1.01, 0.73 | 16.4, 18.1, 16.4 | Reproduction | Maenaka and Park (36) |
The List of Amino Acid Values in Mature Silkworm and Pupae Extracts Detected by the Liquid Chromatograph-Mass Spectrometer Method
| Row | Amino Acids | Pupae (µM) | Silkworm (µM) | Some Roles of Amino Acids | References |
|---|---|---|---|---|---|
| 1 | Histidine (His) | 3293.63 | 2240.192 | Protein methylation, anti-oxidation, vasodilator, regulation of gut function, carrying oxygen | Wang et al. (51); Wu (52) |
| 2 | Serine (Ser) | 1923.89 | 779.811 | Gluconeogenesis and fat metabolism, protein phosphorylation, made of fibroin and sericin, immune system health, muscle growth | Wu (52) |
| 3 | Glutamic acid (Glu) | 1581.03 | 1246.546 | Regulation of protein turnover, gene expression, immune function, cell proliferation, apoptosis inhibition, cell division | Wu (52) |
| 4 | Methionine (Met) | 1439.43 | 225.382 | Synthesis of betaine, acetylcholine, phosphatidylcholine, cellular metabolism, and nutrition | Yoneda et al. (53) |
| 5 | Alanine (Ala) | 1279.13 | 842.012 | Made of fibroin, increases immunity, provides energy for muscle tissue, brain, and CNS | McDonald and Johnston (54) |
| 6 | Glycine (Gly) | 1089.27 | 967.39 | Made of fibroin, an inhibitory neurotransmitter in the CNS | McDonald and Johnston (54) |
| 7 | Lysine (Lys) | 925.07 | 606.08 | Anti-viral activity, muscle growth, and regeneration, Structure, and function of collagen | Wu (52) |
| 8 | Threonine (Thr) | 764.82 | 598.921 | Immune function, and protein phosphorylation, in the construction of elastin, collagen, and the nervous system | McDonald and Johnston (54) |
| 9 | Proline (Pro) | 715.87 | 225.427 | Collagen structure and function, neurological function, killing pathogens, intestinal integrity, immunity | Li and Wu (55); Wang et al. (51) |
| 10 | Valine (Val) | 568.28 | 214.958 | Made of fibroin, synthesis of glutamine and alanine | Wu (52) |
| 11 | Leucine (Leu) | 439.66 | 172.949 | Regulation of protein turnover | Wu (52) |
| 12 | Arginine (Arg) | 410.32 | 305.282 | Anti-oxidant, regulator of hormone secretion, ammonia detoxification, regulation of gene expression, immune function | Yoneda et al. (53) |
| 13 | Tyrosine (Tyr) | 185.62 | 1.928 | Protein phosphorylation, nitrosation, and sulfation, neurotransmitter, regulation of immune response, made of fibroin | Hausler et al. (56) |
| 14 | Isoleucine (Iso) | 151.73 | 29.285 | Diverse physiological functions such as assisting wound healing, detoxification of nitrogenous wastes, stimulating immune function, and promoting secretion of several hormones | Li and Wu (55) |
| 15 | Phenylalanine (Phe) | 138.33 | 461.546 | Neurological development and function | Hausler et al. (56) |
| 16 | Tryptophan (Try) | 69.5 | 38.099 | Neurotransmitter, inhibition production of inflammatory cytokines, and superoxide | Hausler et al. (56); Wu (52) |
| 17 | Citrulline (Cit) | 18.44 | 18.941 | Anti-oxidant, arginine synthesis, ammonia detoxification | Wu (52) |
| 18 | Ornithine (Orn) | 14.62 | 86.573 | Weightlifting supplements wound healing, and sleep quality, play a role in the urea cycle | Wu (52) |
| 19 | Asparagine (Asp) | 8.15 | 62.182 | Cell metabolism and physiology, regulation of gene expression, immune function, ammonia detoxification, the function of the nerve system | Li and Wu (55) |
| 20 | Aspartic acid (ASA) | 0 | 0 | Purine, pyrimidine, asparagine, and arginine synthesis, synthesis of inositol and β-alanine, and urea cycle | Kong et al. (57) |
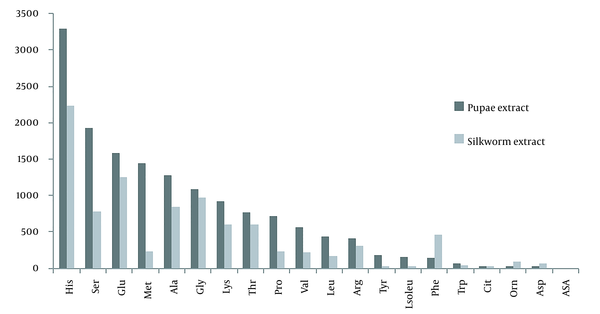
Diagram of amino acid values in mature silkworm and pupae extract based on quantitative values obtained in liquid chromatograph-mass spectrometer (LC-MS/MS)
4.2. Purity of Isolated Schwann Cells
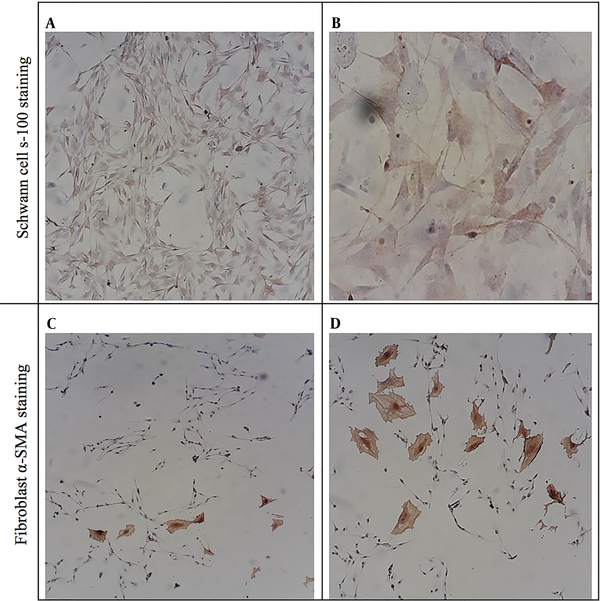
The purity of isolated SCs was proved through immunocytochemical staining against S-100 and alpha-smooth muscle actin (α-SMA), which showed SCs and fibroblast, respectively (Figure 3). To quantify SCs purity, the numbers of S100 and alpha-SMA positive cells were counted, and the purity of SCs was calculated to be > 97%.
Schwann cells (A, B) and fibroblast (C, D) were immunocytochemically stained against S-100 and alpha-smooth muscle actin (α-SMA), respectively. As can be seen in A and B images, the abundance of Schwann cells is noticeable, but in C and D images, the number of fibroblasts is very low.
4.3. Cell Proliferation Assay
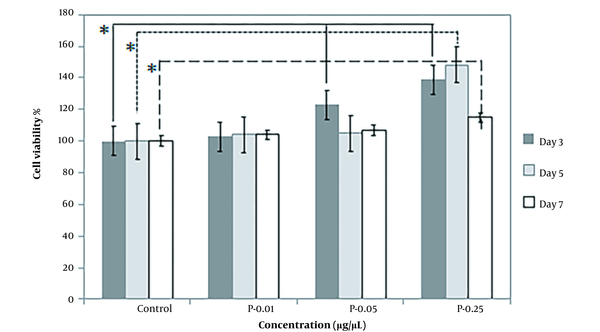
The effect of extracts on SC proliferation was evaluated using an MTT assay. Schwann cells were seeded and treated with mature silkworm and pupae extracts in increasing quantities (0.005, 0.01, 0.05, 0.25, 1.25, 5, and 25 µg/µL) and compared with the control group for three days (Figure 4). No cytotoxicity was found in SCs that were treated with concentrations ranging from 0.01 to 5, although a higher concentration of 25 significantly reduced cell viability by 25 ± 0.003 and 37 ± 0.003% for mature and pupae extracts, respectively. Using both extracts, the percentage of cell viability in groups exposed to 0.01, 0.05, and 0.25 µg/µL was statistically significant compared to the control group (P < 0.05).
The effect of mature silkworm (silkworm extract) and silkworm pupae (pupae extract) extracts on Schwann cell viability. No cytotoxicity was found in Schwann cells that were treated with concentrations ranging from 0.01 to 5 µg/µL, although the higher concentration (25 µg/µL) significantly reduced cell viability by 25 ± 0.003 and 37 ± 0.003% for mature and pupae extracts, respectively. Using both extracts, the percentage of cell viability in groups exposed to 0.01, 0.05, and 0.25 µg/µL was statistically significant compared to the control group. * P < 0.05 (the data were analyzed by one-way ANOVA and post hoc Tukey HSD test).

In addition, the effect of higher potential concentrations (i.e., 0.01, 0.05, 0.25) of both prepared extracts on SC proliferation was compared at different exposure times, including 3, 5, and 7 days. When SCs were treated with varying concentrations of extracts, they proliferated more quicker than controls. In comparison to the control, cell viability increased after treatment with concentrations of 0.01, 0.05, and 0.25. The most effective concentration of mature silkworm and pupae extract was 0.25, especially on the third and fifth days, which increased SC proliferation in all extract groups. When incubated in a medium with 0.25 pupae extract, the percentage of cell viability was the largest (Figures 5 and 6).
The percentage of Schwann cell viability on the third (3 days), fifth (5 days), and seventh (7 days) days of exposure to mature silkworm (S) extract at the concentrations of 0.01, 0.05, and 0.25 µg/µL compared to the control cells cultured in standard cell culture medium (Dulbecco′s Modified Eagle′s Medium (DMEM)/F12 + 10% fetal bovine serum (FBS)). * P < 0.05 (the data were analyzed by one-way ANOVA and post hoc Tukey HSD test).
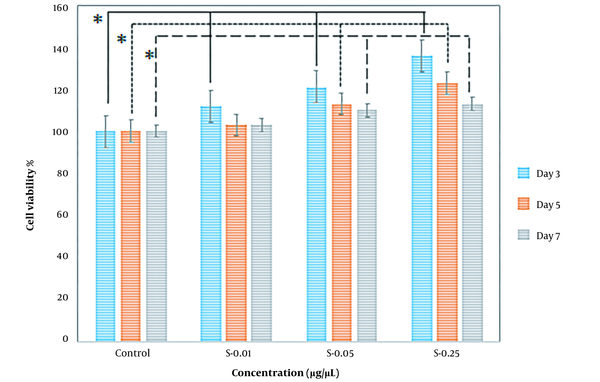
The percentage of Schwann cell viability on the third (3 days), fifth (5 days), and seventh (7 days) days of exposure to pupae extracts (P) at the concentrations of 0.01, 0.05, and 0.25 µg/µL compared to the control cells cultured in standard cell culture medium (Dulbecco′s Modified Eagle′s Medium (DMEM)/F12 + 10% fetal bovine serum (FBS)). * P < 0.05 (the data were analyzed by one-way ANOVA and post hoc Tukey HSD test).
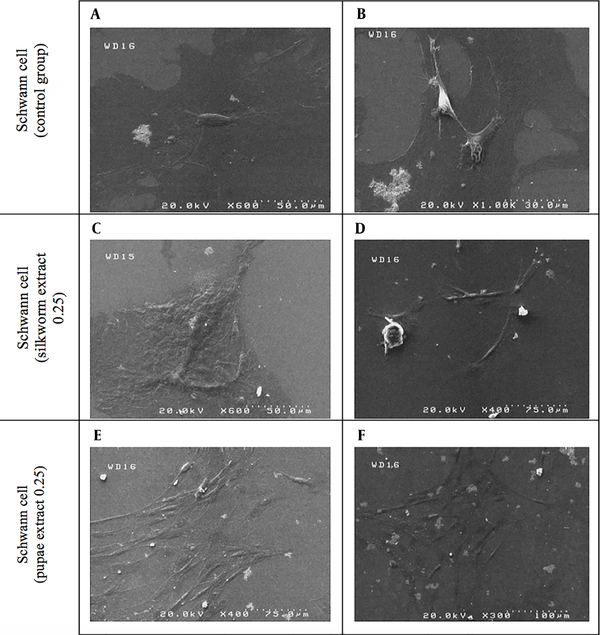
4.4. Morphological Assessment of Schwann Cells
Figure 7 shows the SEM images of SCs cultivated on coverslips and five days of exposure to the effective concentration (0.25 µg/µL) of mature silkworm and pupae extract and compared with the control group.
Scanning electron microscopy (SEM): Schwann cells after exposure to mature silkworm extract (C, D) and pupae extract (E, F) compared to the control group (A and B)
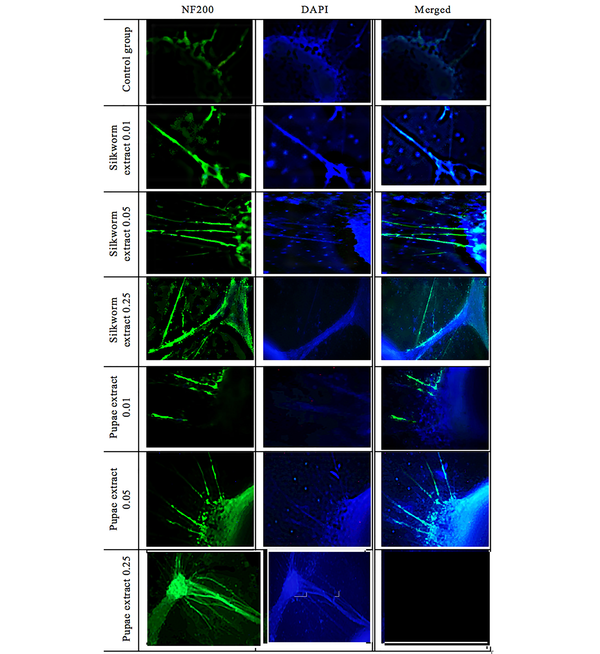
4.5. DRGs Growth
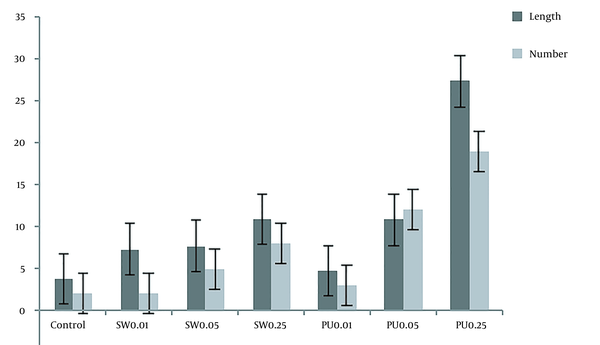
After culturing and affecting adult silkworm and pupae extracts, the DRGs were compared with the control group. To evaluate the results and the effect of the extracts, they were stained with NF-200 and photographed with a fluorescent microscope. Figures 8 and 9, and Table 3 show the significant effect of the extracts on the length and number of axons that outgrew from the DRGs relative to the control group.
Dorsal root ganglions (DRGs) in the control group and exposed to different doses of mature silkworm (0.01, 0.05, and 0.25 µg/µL) and pupae extract (0.01, 0.05, and 0.25 µg/µL) in terms of length and number of axons under a fluorescent microscope
The length and number of axons based on the results obtained by Image J software. In this diagram, the length and number of axons under the influence of mature silkworm (SW: 0.01, 0.05, and 0.25) and pupae (PU: 0.01, 0.05, and 0.25) extracts are compared with each other, and the control group.
The Length and Number of Axons in Dorsal Root Ganglion Images Using Image J Software After Exposure to Mature Silkworm and Pupae Extracts
| DRG Groups | Length of Axons (mm) | Number of Axons |
|---|---|---|
| Control | 3.8 | 2 |
| Silkworm extract (0.01) | 7.3 | 2 |
| Silkworm extract (0.05 mg/mL) | 7.7 | 5 |
| Silkworm extract (0.25 mg/mL) | 10.9 | 8 |
| Pupae extract (0.01 mg/mL) | 4.8 | 3 |
| Pupae extract (0.05 mg/mL) | 10.8 | 12 |
| Pupae 0.25 | 27.3 | 19 |
5. Discussion
Despite the evidence regarding the use of mature silkworm and pupae extracts for enhancing the function of the human reproductive system, to the best of our knowledge, this is the first study conducted on nerve regeneration with the application of mature silkworm and pupae extracts. Also, there are many studies discussing the influence of silkworm cocoons or their products, such as fibroin and sericin as scaffolds for nerve regeneration (27, 31, 58-60); however, there is a gap in knowledge about the effect of proteins extracted from pupae and mature silkworms on nerve regeneration and in this study, we evaluated their effect on SC proliferation and axon growth.
Peripheral nerve injury is a prevalent disorder in clinics that causes a partial loss of segmental motor, sensory, and autonomic functions, as well as significant life and financial difficulties for patients and their families. Numerous researches and clinical observations have reported that some materials, drugs, and products can improve axon regeneration and elongation following peripheral nerve injury. In this study, we evaluated the effect of mature silkworm extract and its pupae to improve their effectiveness on SC proliferation and axon elongation. In the current study, we used mature silkworms and pupae extracts, as they are two important stages in the evolutionary process of silkworms because of the presence of more proteins and growth factors in living insect forms. The study was conducted in two directions. First, laboratory tests to investigate the components of extracts, especially amino acids and proteins, such as Bradford tests, SDS- page, and LC-MS/MS. Then, culturing SCs and DRGs and examining the effect of extracts on them by performing tests, such as MTT, SEM, and immunostaining assay. The average Bradford test results in pupae extract were almost twice that of mature silkworm extract (5 vs. 2.4 mg/mL). In one-dimensional electrophoresis, in terms of molecular weight and optical density of proteins of both extracts, sericin was not detected in mature worm extract. However, the amount of fibroin, ferritin, apolipoprotein, and lipoprotein in mature silkworm extract was higher, and the amount of sericin, glycoprotein, bombyrin, laminin, and arylphorin storage protein in pupae extract was higher. In most cases, the difference was not very large and desirable in both extracts. These proteins play important roles in various fields of biology, biomaterials, biomedical, and regenerative medicine, such as tissue and organ development, immunity, the immune response against various pathogens, reproduction, prolongation of life and inhibition of cell death, cell proliferation, DNA/RNA synthesis, detoxification, nerve growth, and regeneration (Figure 1 and Table 1).
Based on LC-MS/MS, out of 20 types of amino acids in nature, 19 types were detected in both extracts. Only the amount of aspartic acid was not detectable, and its value was zero. Of the 19 amino acids, the amount of 15 amino acids in pupae extract was higher than that of mature silkworm extract. However, in four amino acids, it was the opposite, and the amounts in the mature silkworm extract were higher; however, the difference was not statistically significant. The highest level of amino acids in both extracts belonged to histidine, followed by serine, glutamic acid, methionine, alanine, glycine, lysine, threonine, proline, valine, leucine, arginine, tyrosine, isoleucine, and tryptophan, respectively. But, the amino acids in mature silkworms that were higher than in pupae included phenylalanine, citrulline, ornithine, and asparagine. Asparagine was the lowest amount of amino acid in both extracts, except aspartic acid, which was not detected. The importance of some of the above amino acids is that they play a role in the production and synthesis of some proteins, like fibroin, sericin, laminin, and bombyrin, which play important roles in areas, such as regenerative medicine, tissue engineering of nerves, and other tissues. Some of these roles are listed in Table 2. For example, we can mention roles, such as immune system health, gene expression, cell proliferation, cell division, cell metabolism and nutrition, anti-viral activity, contraction of elastin, collagen, and promoting the secretion of several hormones and neurotransmitters (Figure 2 and Table 2).
In the next part of the study, the effect of different concentrations of extracts on SC proliferation was investigated. Then, three effective concentrations of 0.01, 0.05, and 0.25 µg/µL obtained from both extracts were re-evaluated and compared based on the MTT assay. It was found that the proliferation of SCs at a concentration of 0.25 µg/µL for both extracts was higher than 0.01 and 0.05 µg/µL. However, at concentrations of 0.01 and 0.05 µg/µL, we also found SC proliferation. The highest SC proliferation was at the concentration of 0.25 µg/µL of pupae on the fifth day then on the third day. On the seventh day, we had less cell viability. In the case of mature silkworm extract, the highest viability was first on the third day and then on the fifth day at a concentration of 0.25 µg/µL compared to the control group. Accordingly, it can be concluded that the highest viability for both extracts was at a concentration of 0.25 µg/µL on the third and fifth days based on the MTT assay (Figures 4, 5 and 6). The effect of extracts on SC morphology on the fifth day was also evaluated. At the concentrations of 0.25 µg/µL, both extracts showed an increase in the SC population compared to the control group (Figure 7).
In the case of DRGs and to evaluate the effect of extracts on outgrowth and the number of axons after exposure to both extracts and comparison with the control group (Figure 8), significant changes were observed. Both adult silkworms (Figure 8) and pupal extracts (Figure 8) increased the length and number of axons. But this increase was more pronounced in DRGs that were affected by pupae extract (Figure 9 and Table 3). Similar studies have indicated that several herbal compounds have a beneficial role in nerve regeneration. Achyranthes bidentata could be a well-known Chinese complementary pharmaceutical with numerous capacities and impacts. A survey showed that the Achyranthes bidentata polypeptides at 0.5 μg/mL for 24 h could significantly promote the proliferation of primary SCs cultured in vitro at different times (61). The co-culture of SCs and adrenal pheochromocytoma (PC-12) cells showed a high cell proliferation rate and axonal expansion (62). Also, Heregulin (10 nM) for 24 h showed a successful mitogen for human SCs (63). The effectiveness of essential disconnected SCs with curcumin (0.25 µM) in ethanol 100% was evaluated for the alleviation of human-related peripheral neuropathies (64). In a study, the effect of the male silkworm extracts on the reproductive tract of male rats was studied. Sperm counts were notably greater in male rats treated with male silkworm larvae extract and male silkworm pupae extract compared to the control group (65). The animal examination showed that alcohol dehydrogenase (ADH) activity was higher in the liver of mice orally administered with 0.5 mg/mL of silkworm extract and alcohol, indicating that silkworm pupae extracts have alcohol detoxification activity (66).
5.1. Conclusions
Although the silk cocoons and some of their proteins have been used in the past in various fields, such as medicine, pharmacy, and textile, the results of the present study showed that the extracts obtained from some stages of the silkworm growth cycle were more effective in nerve regeneration and repair. This study focused on the effect of extracts from pupae and mature silkworms on SC proliferation and axon growth. The optimized concentration of both extracts on cell proliferation and axon growth was determined, and it was found that at the same concentration, the pupae extract was more effective on SC proliferation and axon growth. Our findings showed that using both extracts can be a suitable candidate for nerve regeneration and repair; however, further investigation is needed to reveal the effect of these extracts in a preclinical study.
Acknowledgements
References
-
1.
Houshyar S, Bhattacharyya A, Shanks R. Peripheral Nerve Conduit: Materials and Structures. ACS Chem Neurosci. 2019;10(8):3349-65. [PubMed ID: 31273975]. https://doi.org/10.1021/acschemneuro.9b00203.
-
2.
Wang L, Lu C, Yang S, Sun P, Wang Y, Guan Y, et al. A fully biodegradable and self-electrified device for neuroregenerative medicine. Sci Adv. 2020;6(50):eabc6686. [PubMed ID: 33310851]. [PubMed Central ID: PMC7732202]. https://doi.org/10.1126/sciadv.abc6686.
-
3.
Nocera G, Jacob C. Mechanisms of Schwann cell plasticity involved in peripheral nerve repair after injury. Cell Mol Life Sci. 2020;77(20):3977-89. [PubMed ID: 32277262]. [PubMed Central ID: PMC7532964]. https://doi.org/10.1007/s00018-020-03516-9.
-
4.
Chen J, Ren S, Duscher D, Kang Y, Liu Y, Wang C, et al. Exosomes from human adipose-derived stem cells promote sciatic nerve regeneration via optimizing Schwann cell function. J Cell Physiol. 2019;234(12):23097-110. [PubMed ID: 31124125]. https://doi.org/10.1002/jcp.28873.
-
5.
Martyn GV, Shurin GV, Keskinov AA, Bunimovich YL, Shurin MR. Schwann cells shape the neuro-immune environs and control cancer progression. Cancer Immunol Immunother. 2019;68(11):1819-29. [PubMed ID: 30607548]. https://doi.org/10.1007/s00262-018-02296-3.
-
6.
Monje PV. Schwann Cell Cultures: Biology, Technology and Therapeutics. Cells. 2020;9(8):1848. [PubMed ID: 32781699]. [PubMed Central ID: PMC7465416]. https://doi.org/10.3390/cells9081848.
-
7.
Filipe ACTM. Characterization of axon-Schwann cell interactions implicated in neuronal energy metabolism [master's thesis]. Porto: University of Porto; 2018.
-
8.
Frob F, Wegner M. The role of chromatin remodeling complexes in Schwann cell development. Glia. 2020;68(8):1596-603. [PubMed ID: 31837180]. https://doi.org/10.1002/glia.23766.
-
9.
Monje PV. The properties of human Schwann cells: Lessons from in vitro culture and transplantation studies. Glia. 2020;68(4):797-810. [PubMed ID: 32027424]. https://doi.org/10.1002/glia.23793.
-
10.
Bolivar S, Navarro X, Udina E. Schwann Cell Role in Selectivity of Nerve Regeneration. Cells. 2020;9(9):2131. [PubMed ID: 32962230]. [PubMed Central ID: PMC7563640]. https://doi.org/10.3390/cells9092131.
-
11.
Liu H, Lv P, Wu H, Zhang K, Xu F, Zheng L, et al. The Proliferation Enhancing Effects of Salidroside on Schwann Cells In Vitro. Evid Based Complement Alternat Med. 2017;2017:4673289. [PubMed ID: 28680451]. [PubMed Central ID: PMC5478829]. https://doi.org/10.1155/2017/4673289.
-
12.
Hopf A, Schaefer DJ, Kalbermatten DF, Guzman R, Madduri S. Schwann Cell-Like Cells: Origin and Usability for Repair and Regeneration of the Peripheral and Central Nervous System. Cells. 2020;9(9):1990. [PubMed ID: 32872454]. [PubMed Central ID: PMC7565191]. https://doi.org/10.3390/cells9091990.
-
13.
Lotfi L, Khakbiz M, Moosazadeh Moghaddam M, Bonakdar S. A biomaterials approach to Schwann cell development in neural tissue engineering. J Biomed Mater Res A. 2019;107(11):2425-46. [PubMed ID: 31254439]. https://doi.org/10.1002/jbm.a.36749.
-
14.
He J, Sun C, Gu Z, Yang Y, Gu M, Xue C, et al. Morphology, Migration, and Transcriptome Analysis of Schwann Cell Culture on Butterfly Wings with Different Surface Architectures. ACS Nano. 2018;12(10):9660-8. [PubMed ID: 30125084]. https://doi.org/10.1021/acsnano.8b00552.
-
15.
Fornasari BE, El Soury M, Nato G, Fucini A, Carta G, Ronchi G, et al. Fibroblasts Colonizing Nerve Conduits Express High Levels of Soluble Neuregulin1, a Factor Promoting Schwann Cell Dedifferentiation. Cells. 2020;9(6):1366. [PubMed ID: 32492853]. [PubMed Central ID: PMC7349576]. https://doi.org/10.3390/cells9061366.
-
16.
Zhu H, Xue C, Yao M, Wang H, Zhang P, Qian T, et al. miR-129 controls axonal regeneration via regulating insulin-like growth factor-1 in peripheral nerve injury. Cell Death Dis. 2018;9(7):720. [PubMed ID: 29915198]. [PubMed Central ID: PMC6006361]. https://doi.org/10.1038/s41419-018-0760-1.
-
17.
Yi S, Liu Q, Wang X, Qian T, Wang H, Zha G, et al. Tau modulates Schwann cell proliferation, migration and differentiation following peripheral nerve injury. J Cell Sci. 2019;132(6):jcs222059. [PubMed ID: 30782778]. https://doi.org/10.1242/jcs.222059.
-
18.
Kim HS, Kim JY, Song CL, Jeong JE, Cho YS. Directly induced human Schwann cell precursors as a valuable source of Schwann cells. Stem Cell Res Ther. 2020;11(1):257. [PubMed ID: 32586386]. [PubMed Central ID: PMC7318441]. https://doi.org/10.1186/s13287-020-01772-x.
-
19.
Mobini S, Kuliasha CA, Siders ZA, Bohmann NA, Jamal SM, Judy JW, et al. Microtopographical patterns promote different responses in fibroblasts and Schwann cells: A possible feature for neural implants. J Biomed Mater Res A. 2021;109(1):64-76. [PubMed ID: 32419308]. [PubMed Central ID: PMC8059778]. https://doi.org/10.1002/jbm.a.37007.
-
20.
Panzer KV, Burrell JC, Helm KVT, Purvis EM, Zhang Q, Le AD, et al. Tissue Engineered Bands of Bungner for Accelerated Motor and Sensory Axonal Outgrowth. Front Bioeng Biotechnol. 2020;8:580654. [PubMed ID: 33330416]. [PubMed Central ID: PMC7714719]. https://doi.org/10.3389/fbioe.2020.580654.
-
21.
Hu X, Hou H, Bastian C, He W, Qiu S, Ge Y, et al. BACE1 regulates the proliferation and cellular functions of Schwann cells. Glia. 2017;65(5):712-26. [PubMed ID: 28191691]. [PubMed Central ID: PMC5357169]. https://doi.org/10.1002/glia.23122.
-
22.
Kamil K, Yazid MD, Idrus RBH, Kumar J. Hydroxytyrosol Promotes Proliferation of Human Schwann Cells: An In Vitro Study. Int J Environ Res Public Health. 2020;17(12):4404. [PubMed ID: 32575426]. [PubMed Central ID: PMC7344605]. https://doi.org/10.3390/ijerph17124404.
-
23.
Aquino JB, Sierra R. Schwann cell precursors in health and disease. Glia. 2018;66(3):465-76. [PubMed ID: 29124786]. https://doi.org/10.1002/glia.23262.
-
24.
Cerqueira SR, Lee YS, Cornelison RC, Mertz MW, Wachs RA, Schmidt CE, et al. Decellularized peripheral nerve supports Schwann cell transplants and axon growth following spinal cord injury. Biomaterials. 2018;177:176-85. [PubMed ID: 29929081]. [PubMed Central ID: PMC6034707]. https://doi.org/10.1016/j.biomaterials.2018.05.049.
-
25.
Marquardt LM, Doulames VM, Wang AT, Dubbin K, Suhar RA, Kratochvil MJ, et al. Designer, injectable gels to prevent transplanted Schwann cell loss during spinal cord injury therapy. Sci Adv. 2020;6(14):eaaz1039. [PubMed ID: 32270042]. [PubMed Central ID: PMC7112763]. https://doi.org/10.1126/sciadv.aaz1039.
-
26.
Anderson KD, Guest JD, Dietrich WD, Bartlett Bunge M, Curiel R, Dididze M, et al. Safety of Autologous Human Schwann Cell Transplantation in Subacute Thoracic Spinal Cord Injury. J Neurotrauma. 2017;34(21):2950-63. [PubMed ID: 28225648]. https://doi.org/10.1089/neu.2016.4895.
-
27.
Yonesi M, Garcia-Nieto M, Guinea GV, Panetsos F, Perez-Rigueiro J, Gonzalez-Nieto D. Silk Fibroin: An Ancient Material for Repairing the Injured Nervous System. Pharmaceutics. 2021;13(3):429. [PubMed ID: 33806846]. [PubMed Central ID: PMC8004633]. https://doi.org/10.3390/pharmaceutics13030429.
-
28.
Sharma A, Gupta RK, Sharma P, Duwa, Attri K, Bandral RS, et al. Silkworm as an edible insect: A review. Pharma Innov J. 2022;11(2S):1667-74.
-
29.
Khorasani MHA. [Makhzan-Al-Adawiyah]. Kolkata, India: Calcutta; 1844. Persian.
-
30.
Momen M. [Tahfato-Al-Momenin]. Kolkata, India: Calcutta; 1669. Persian.
-
31.
Khosropanah MH, Alizadeh Vaghasloo M, Shakibaei M, Mueller AL, Kajbafzadeh AM, Amani L, et al. Biomedical applications of silkworm (Bombyx Mori) proteins in regenerative medicine (a narrative review). J Tissue Eng Regen Med. 2022;16(2):91-109. [PubMed ID: 34808032]. https://doi.org/10.1002/term.3267.
-
32.
Millesi F, Weiss T, Mann A, Haertinger M, Semmler L, Supper P, et al. Defining the regenerative effects of native spider silk fibers on primary Schwann cells, sensory neurons, and nerve-associated fibroblasts. FASEB J. 2021;35(2). e21196. [PubMed ID: 33210360]. [PubMed Central ID: PMC7894153]. https://doi.org/10.1096/fj.202001447R.
-
33.
Debastiani JC, Santana AJ, Ribeiro LFC, Brancalhao RMC, Bertolini GRF. Sericin silk protein in peripheral nervous repair associated with the physical exercise of swimming in Wistar rats. Neurol Res. 2019;41(4):326-34. [PubMed ID: 30638158]. https://doi.org/10.1080/01616412.2018.1564187.
-
34.
Li X, Zhang Q, Luo Z, Yan S, You R. Biofunctionalized silk fibroin nanofibers for directional and long neurite outgrowth. Biointerphases. 2019;14(6):61001. [PubMed ID: 31731836]. https://doi.org/10.1063/1.5120738.
-
35.
Ebrahimi M, Ai J, Biazar E, Ebrahimi-Barough S, Khojasteh A, Yazdankhah M, et al. In vivo assessment of a nanofibrous silk tube as nerve guide for sciatic nerve regeneration. Artif Cells Nanomed Biotechnol. 2018;46(sup1):394-401. [PubMed ID: 29336177]. https://doi.org/10.1080/21691401.2018.1426593.
-
36.
Maenaka K, Park EY. Silkworm Biofactory: Silk to Biology. Boca Raton: CRC Press; 2018. https://doi.org/10.1201/9780429448881.
-
37.
Kim HJ, Je HJ, Cheon HM, Kong SY, Han J, Yun CY, et al. Accumulation of 23 kDa lipocalin during brain development and injury in Hyphantria cunea. Insect Biochem Mol Biol. 2005;35(10):1133-41. [PubMed ID: 16102419]. https://doi.org/10.1016/j.ibmb.2005.05.004.
-
38.
Rupprecht R, Papadopoulos V, Rammes G, Baghai TC, Fan J, Akula N, et al. Translocator protein (18 kDa) (TSPO) as a therapeutic target for neurological and psychiatric disorders. Nat Rev Drug Discov. 2010;9(12):971-88. [PubMed ID: 21119734]. https://doi.org/10.1038/nrd3295.
-
39.
Krames ES. The dorsal root ganglion in chronic pain and as a target for neuromodulation: a review. Neuromodulation. 2015;18(1):24-32. discussion 32. [PubMed ID: 25354206]. https://doi.org/10.1111/ner.12247.
-
40.
Ventre DM, Cluff A, Gagnon C, Diaz Vera D, Koppes RA, Koppes AN. The effects of low intensity focused ultrasonic stimulation on dorsal root ganglion neurons and Schwann cells in vitro. J Neurosci Res. 2021;99(1):374-91. [PubMed ID: 32743823]. https://doi.org/10.1002/jnr.24700.
-
41.
Dionigi C, Posati T, Benfenati V, Sagnella A, Pistone A, Bonetti S, et al. A nanostructured conductive bio-composite of silk fibroin-single walled carbon nanotubes. J Mater Chem B. 2014;2(10):1424-31. [PubMed ID: 32261458]. https://doi.org/10.1039/c3tb21172j.
-
42.
Firouzi M, Sabouni F, Deezagi A, Hassannejad Pirbasti Z, Poorrajab F, Rahimi-Movaghar V. Schwann cell apoptosis and p75(NTR) siRNA. Iran J Allergy Asthma Immunol. 2011;10(1):53-9. [PubMed ID: 21358016].
-
43.
Hopkins AM, De Laporte L, Tortelli F, Spedden E, Staii C, Atherton TJ, et al. Silk Hydrogels as Soft Substrates for Neural Tissue Engineering. Adv Funct Mater. 2013;23(41):5140-9. https://doi.org/10.1002/adfm.201300435.
-
44.
Endo T, Kadoya K, Kawamura D, Iwasaki N. Evidence for cell-contact factor involvement in neurite outgrowth of dorsal root ganglion neurons stimulated by Schwann cells. Exp Physiol. 2019;104(10):1447-54. [PubMed ID: 31294871]. https://doi.org/10.1113/EP087634.
-
45.
Entekhabi E, Haghbin Nazarpak M, Shafieian M, Mohammadi H, Firouzi M, Hassannejad Z. Fabrication and in vitro evaluation of 3D composite scaffold based on collagen/hyaluronic acid sponge and electrospun polycaprolactone nanofibers for peripheral nerve regeneration. J Biomed Mater Res A. 2021;109(3):300-12. [PubMed ID: 32490587]. https://doi.org/10.1002/jbm.a.37023.
-
46.
Dai ZJ, Sun W, Zhang Z. Comparative analysis of iTRAQ-based proteomes for cocoons between the domestic silkworm (Bombyx mori) and wild silkworm (Bombyx mandarina). J Proteomics. 2019;192:366-73. [PubMed ID: 30287406]. https://doi.org/10.1016/j.jprot.2018.09.017.
-
47.
Dong Z, Zhao P, Zhang Y, Song Q, Zhang X, Guo P, et al. Analysis of proteome dynamics inside the silk gland lumen of Bombyx mori. Sci Rep. 2016;6:21158. [PubMed ID: 27102218]. [PubMed Central ID: PMC4840313]. https://doi.org/10.1038/srep21158.
-
48.
Chandrasekar R, Habeeb SKM. Focus on Physiological Vital Proteins from Silkworm Bombyx mori. In: Maenaka K, Park EY, editors. Silkworm Biofactory: Silk to Biology. Boca Raton: CRC Press; 2018. p. 38-61.
-
49.
Asakura T, Isozaki M, Saotome T, Tatematsu KI, Sezutsu H, Kuwabara N, et al. Recombinant silk fibroin incorporated cell-adhesive sequences produced by transgenic silkworm as a possible candidate for use in vascular graft. J Mater Chem B. 2014;2(42):7375-83. [PubMed ID: 32261962]. https://doi.org/10.1039/c4tb01301h.
-
50.
Tomita M. Production of Medical and Cosmetic Products using Transgenic Silkworms. In: Maenaka K, Park EY, editors. Silkworm Biofactory: Silk to Biology. Boca Raton: CRC Press; 2018. p. 251-65.
-
51.
Wang WW, Qiao SY, Li DF. Amino acids and gut function. Amino Acids. 2009;37(1):105-10. [PubMed ID: 18670730]. https://doi.org/10.1007/s00726-008-0152-4.
-
52.
Wu G. Amino acids: metabolism, functions, and nutrition. Amino Acids. 2009;37(1):1-17. [PubMed ID: 19301095]. https://doi.org/10.1007/s00726-009-0269-0.
-
53.
Yoneda J, Andou A, Takehana K. Regulatory Roles of Amino Acids in Immune Response. Curr Rheumatol Rev. 2009;5(4):252-8. https://doi.org/10.2174/157339709790192567.
-
54.
McDonald JW, Johnston MV. Physiological and pathophysiological roles of excitatory amino acids during central nervous system development. Brain Res Brain Res Rev. 1990;15(1):41-70. [PubMed ID: 2163714]. https://doi.org/10.1016/0165-0173(90)90011-c.
-
55.
Li P, Wu G. Important roles of amino acids in immune responses. Br J Nutr. 2022;127(3):398-402. [PubMed ID: 34776020]. https://doi.org/10.1017/S0007114521004566.
-
56.
Hausler RE, Ludewig F, Krueger S. Amino acids--a life between metabolism and signaling. Plant Sci. 2014;229:225-37. [PubMed ID: 25443849]. https://doi.org/10.1016/j.plantsci.2014.09.011.
-
57.
Kong X, Wu G, Yin Y. Roles of phytochemicals in amino acid nutrition. Front Biosci (Schol Ed). 2011;3(1):372-84. [PubMed ID: 21196382]. https://doi.org/10.2741/s157.
-
58.
Banagozar Mohammadi A, Sadigh-Eteghad S, Torbati M, Bagher Fazljou SM, Vatandoust SM, Ej Golzari S, et al. Identification and applications of neuroactive silk proteins: a narrative review. J Appl Biomed. 2019;17(3):147-56. [PubMed ID: 34907702]. https://doi.org/10.32725/jab.2019.012.
-
59.
Radtke C. Natural Occurring Silks and Their Analogues as Materials for Nerve Conduits. Int J Mol Sci. 2016;17(10):1754. [PubMed ID: 27775616]. [PubMed Central ID: PMC5085779]. https://doi.org/10.3390/ijms17101754.
-
60.
Dinis TM, Vidal G, Jose RR, Vigneron P, Bresson D, Fitzpatrick V, et al. Complementary effects of two growth factors in multifunctionalized silk nanofibers for nerve reconstruction. PLoS One. 2014;9(10). e109770. [PubMed ID: 25313579]. [PubMed Central ID: PMC4196919]. https://doi.org/10.1371/journal.pone.0109770.
-
61.
Tang L, Zhang M, Liu X, Zhu Y, Chen X, Zhong J, et al. Effects and molecular mechanisms of Achyranthes bidentata polypeptide k on proliferation of Schwann cells. Ann Transl Med. 2021;9(20):1581. [PubMed ID: 34790787]. [PubMed Central ID: PMC8576723]. https://doi.org/10.21037/atm-21-5181.
-
62.
Huang Y, Xu K, Liu J, Dai G, Yin J, Wei P. Promotion of Adrenal Pheochromocytoma (PC-12) Cell Proliferation and Outgrowth Using Schwann Cell-Laden Gelatin Methacrylate Substrate. Gels. 2022;8(2):84. [PubMed ID: 35200467]. [PubMed Central ID: PMC8871842]. https://doi.org/10.3390/gels8020084.
-
63.
Levi AD, Bunge RP, Lofgren JA, Meima L, Hefti F, Nikolics K, et al. The influence of heregulins on human Schwann cell proliferation. J Neurosci. 1995;15(2):1329-40. [PubMed ID: 7869101]. [PubMed Central ID: PMC6577846]. https://doi.org/10.1523/JNEUROSCI.15-02-01329.1995.
-
64.
Vazquez Alberdi L, Rosso G, Veloz L, Romeo C, Farias J, Di Tomaso MV, et al. Curcumin and Ethanol Effects in Trembler-J Schwann Cell Culture. Biomolecules. 2022;12(4):515. [PubMed ID: 35454103]. [PubMed Central ID: PMC9025918]. https://doi.org/10.3390/biom12040515.
-
65.
Kwon MG, Kim DS, Lee JH, Park SR, Ahn J, Kim JS, et al. Enhanced activities of reproductive system in male rat treated with male silkworm pupae extract. Entomol Res. 2013;43(2):101-7. https://doi.org/10.1111/1748-5967.12011.
-
66.
Kwon MG, Kim DS, Lee JH, Park SW, Choo YK, Han YS, et al. Isolation and analysis of natural compounds from silkworm pupae and effect of its extracts on alcohol detoxification. Entomol Res. 2012;42(1):55-62. https://doi.org/10.1111/j.1748-5967.2011.00439.x.
