1. Background
Severe deprivation of oxygen and nutrients in the liver due to hemorrhages and arterial occlusions or during transplantation results in hepatic ischemia, and the subsequent reperfusion paradoxically exacerbates the tissue injury. Ischemia/reperfusion injury is a despairing concern, particularly for the transplantation recipients who wait on the list for about two months in the UK (1) and about four months in Turkey (2). Despite the advantages of living donor liver transplantation (LDLT) (e.g., higher availability, fewer complications, rejections, and healthcare costs) over deceased donor liver transplantation (DDLT), living donations of the liver constitute a small portion of all donations in individualistic societies (< 5%) unlike collective ones in which an opposite trend prevails (> 50%) (3-6). Moreover, DDLT is more susceptible to ischemia/reperfusion injury due to the occurrence of prolonged ischemia as well as systemic failures and inflammation in the terminal phase of the donor’s life (7, 8).
Ischemia/reperfusion-induced inflammation is closely related to oxidative stress. Reactive oxygen species promote inflammation and vice versa; inflammation aggravates the production of reactive oxygen species, archetypally creating the self-devouring serpent of Ouroboros which leads to the failure of vital function (9-11). Several transcription regulators that take part in the inflammatory response have much in common with oxidative stress, including RelA (nuclear factor-κB [NF-κB] p65 subunit), IκB (inhibitor factor-κB), PPARs (peroxisome proliferator-activated receptors), and CREBs (cAMP responsive element binding proteins), which may connect inflammation to oxidative stress (12-14). RelA and IκB both play a central role in inflammation. The complex of RelA and IκB remains silent in the cytoplasm until a proinflammatory stimulus (canonical pathway) degrades IκB and allows RelA to promote inflammatory gene transcription (15, 16). Likewise, oxidative stress stimulates this pathway by inhibiting IκB and leads to inflammation, but indeed, it is now to make use of RelA-induced antioxidants (17). Meanwhile, PPAR-α and PPAR-γ which possess an anti-inflammatory function through inhibiting RelA are suppressed by both inflammation and oxidative stress (12, 18, 19). Another inhibitor of RelA signaling is CREB1, also known as CREB, which competes with the co-activator of RelA, namely p300/CBP (20). In addition, CREB1 provokes the activation of PPARs (21).
Turmeric (Curcuma longa L.), with its major polyphenol, curcumin has a long history of application as a medicinal plant that goes back to about 4000 years (22). Curcumin is one of the most potent antioxidant phenolic compounds (23) and is believed to have beneficial effects on diabetes, neurodegeneration, neoplasms, hypertension, and autoimmune diseases, as well as ischemia/reperfusion injury (24-29).
2. Objectives
Taking into account the hypothesized links between the above-mentioned inflammatory pathways, the present study aimed to investigate the role of PPARs and CREB1 as well as to elucidate the possible pathway to the hepatoprotective features of curcumin in an experimental hepatic ischemia/reperfusion setting.
3. Methods
3.1. Chemicals
Curcumin and corn oil were obtained from Sigma-Aldrich (St. Louis, MO, USA) and Kucukbay A.S. (Izmir, Turkey), respectively. Suppliers of other chemicals used in the analyses are introduced in respective sections.
3.2. Modelling of Hepatic Ischemia/Reperfusion
Adult male Wistar albino rats were assigned to four groups (n = 8 for each) as Control (only vehicle), Sham (sham surgery and vehicle), I/R (hepatic ischemia/reperfusion and vehicle), and Cur+I/R (hepatic ischemia/reperfusion and curcumin). The animals were housed under standard laboratory conditions and provided with ad libitum rat chow. Control, Sham, and I/R groups received corn oil (vehicle; 1 mL/day, p.o.), whereas 50 mg/kg/day, p.o. of curcumin was orally administered to Cur+I/R animals for 14 days. Afterwards, all animals were anesthetized using a combination of ketamine and xylazine (80/12 mg/kg, i.p.), which was supplemented with 27 mg/kg of ketamine on an hourly basis to extend anesthesia. Controls were sacrificed at the 180th minute of anesthesia by exsanguination. Following appropriate surgical asepsis, a midline laparotomy was performed on a heating pad set to 37°C, and the liver was exposed in Sham, I/R, and Cur+I/R animals. In the Sham group, the portal triad was only explored while it was clamped with an atraumatic Bulldog vascular clamp to generate global ischemia in I/R and Cur+I/R animals. Then the incision was temporarily sutured, and after 60 minutes of ischemia, the abdomen was re-opened, and the clamp was removed to initiate 120-minute reperfusion. Subsequently, the animals were sacrificed, and the liver was excised and perfused with saline. Slices of the hepatic tissue were either frozen for molecular analyses or fixed in 10% neutral buffered formalin and embedded in paraffin for histological examination.
Ischemia for 60 minutes is the upper limit for reversible hepatic injury, and longer durations result in irreparable damage in rat (30). Initial response to reperfusion is apoptotic activation in early phase (31). Afterwards, hepatocellular necrosis and consequent hepatic dysfunction gain prominence, which complicates pathophysiology and, of course, experimental findings (31, 32). Similar to the study by Tian et al. (33), therefore, 60 minutes of ischemia and 120 minutes of reperfusion were preferred in the present study to reach translatable and replicable results.
3.3. Sample Preparation and Biochemical Analyses
Blood was poured into a serum separator tube with clot activator gel. After centrifugation, serum was collected for biochemical tests. Serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), and total and conjugated bilirubin concentrations were estimated using an automated analyzer (Siemens, Erlangen, Germany). The liver tissue was homogenized in either a protein-preserving (Thermo Scientific, Waltham, MA, USA) or an RNA-preserving lysis solution (Qiagen, Hilden, Germany) with a temperature-controlled ultrasonic homogenizer (JP Selecta, Barcelona, Spain). After centrifugation, the supernatant was collected. Total protein concentration was estimated spectrophotometrically at 450 nm by adopting the Bradford method for protein detections; RNA was isolated using a commercial kit (Qiagen, Hilden, Germany) followed by total RNA measurement at 260 nm with background subtraction (320 nm) for genetic analyses.
3.4. Western Blotting
Samples containing 25 mg of total protein were loaded onto a stain-free polyacrylamide gel (Bio-Rad, Hercules, CA, USA). After the electrophoresis at 80 mV for ~5 minutes and at 180 mV for ~60 minutes, the gel was UV-activated, and the proteins were transferred to a polyvinylidene fluoride (PVDF) membrane using a semi-dry blotter (Bio-Rad, Hercules, CA, USA). The membrane was blocked with 8% skim milk for 1.5 hours and, then, was incubated overnight using the primary antibody against RelA or IκB (1:1000 dilution; Invitrogen, Carlsbad, CA, USA) at 4°C and with secondary antibody (1:10000 dilution; Advansta, Menlo Park, CA, USA) for 1.5 hours at the room temperature. Following the treatment with enhanced chemiluminescence (ECL) solution, band-specific signals were acquired by using an imaging system (Bio-Rad, Hercules, CA, USA). Optical densities were normalized against the total protein signal of respective lane.
3.5. Quantitative Polymerase Chain Reaction
cDNA was synthesized by using a commercial reverse transcriptase kit according to instructions of the manufacturer (Applied Biosystems, Foster City, CA, USA). Target mRNA was amplified with a mix of primer/probe (Applied Biosystems, Foster City, CA, USA) for PPAR-α (Ref: Rn00566193_m1), PPAR-γ (Ref: Mm00440940_m1), and CREB1 (Ref: Rn00578829_g1) on a real-time cycler (Qiagen, Hilden, Germany). The cycle consisted of initialization at 95°C for 10 minutes followed by forty-five thermal cycles at 95°C for 15 seconds and 60°C for 1 minute. The expression was normalized in reference to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and calculated using 2-∆∆Ct method.
3.6. Histological Examination
Paraffin blocks with a thickness of 5 µm were sectioned, and the slides were stained with hematoxylin for 5 min after de-paraffinization in xylene and rehydration in graded alcohol and then were counterstained with eosin for 2 min at the room temperature. After washing and clearing, the slides were examined using a wide-field microscope (Olympus, Tokyo, Japan) with 400x magnification.
3.7. Statistical Analyses
The data, which were non-parametric according to normality and homogeneity inspection, were analyzed by performing Kruskal-Wallis and post-hoc Dunn’s tests and using Prism v.8 software (Graphpad, San Diego, CA, USA). The results were reported as medians ± interquartile range for biochemical, fold-change for Western blot, and log fold-change for qPCR data. The statistical significance level was set at P < 0.05.
4. Results
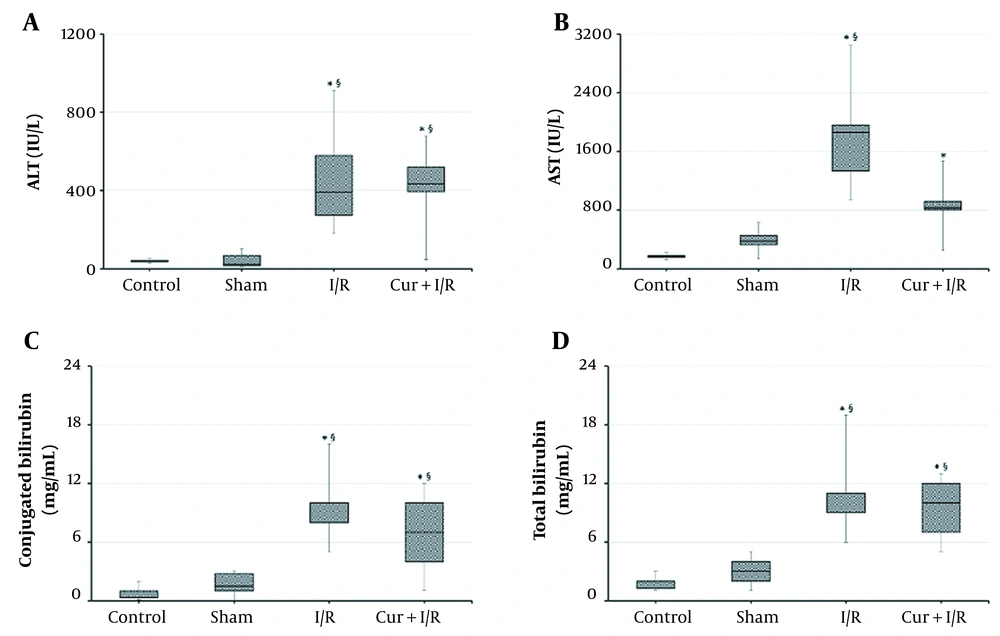
According to the results from liver function tests, ALT and AST were increased in I/R animals compared to both controls (P = 0.002 and P < 0.001, respectively) and the Sham group (P < 0.001 and P = 0.001, respectively). Likewise, ALT was higher in Cur+I/R group than that in controls (P = 0.003), and Sham animals (P = 0.001), but AST was significant only against controls (P = 0.004). There was no significant difference between Control and Sham groups regarding the liver aminotransferases (P > 0.05) (Figure 1A and B). Total and conjugated bilirubin concentrations were elevated in I/R (P < 0.001 and P < 0.001, respectively) and Cur+I/R groups (P < 0.001 and P = 0.004, respectively) in comparison to controls, and a similar difference was found in I/R (P = 0.003 and P < 0.001, respectively) and Cur+I/R animals (P = 0.006 and P = 0.044, respectively) compared to Sham group (Figure 1C and D).
Serum Aminotransferase and Bilirubin Concentrations. Ischemia/reperfusion resulted in elevated liver aminotransferases and bilirubin, regardless of the supplementation of curcumin. Asterisk (*) shows statistical significance (P < 0.05; Kruskal-Wallis and post-hoc Dunn’s tests) vs Control, and section sign (§) vs Sham. ALT: Alanine aminotransferase. AST: Aspartate aminotransferase.
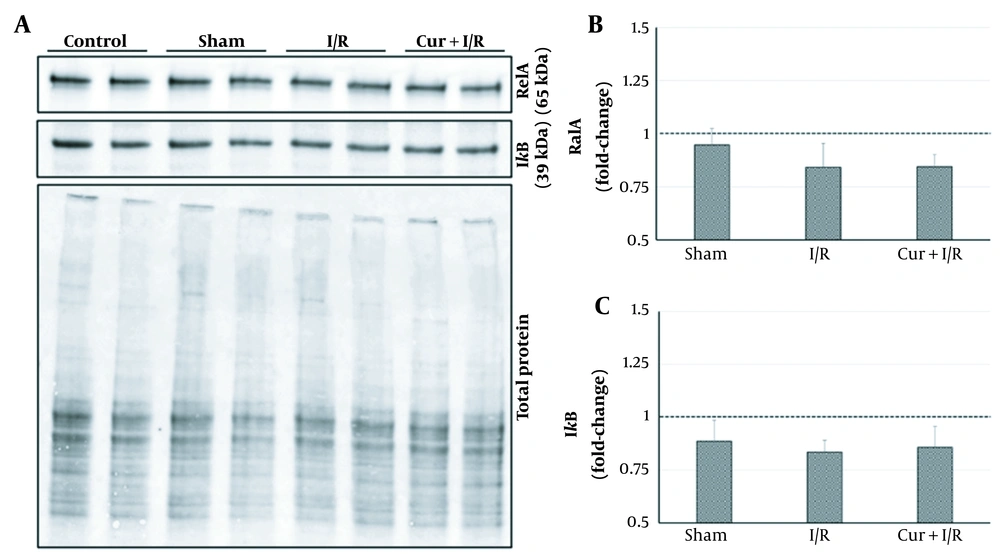
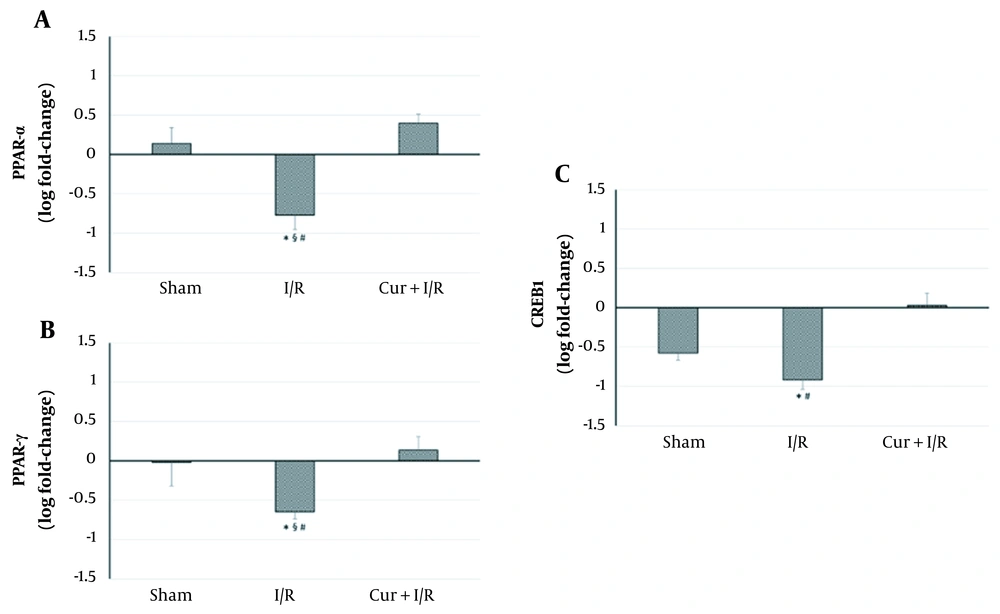
As shown on the blot in Figure 2, expressions of the key regulators of inflammation, RelA, and IκB, were similar in the groups (P > 0.05); in other words, neither sham operation and ischemia/reperfusion nor curcumin supplementation in ischemia/reperfusion-generated subjects altered their expression. However, expressions of the inflammation-modulating transcription factors of PPAR-α and PPAR-γ were significantly reduced in I/R group compared to both controls (P = 0.027 and P = 0.016, respectively) and Sham animals (P = 0.016 and P = 0.032, respectively). PPAR-α and PPAR-γ expressions exceeded the levels of controls after using curcumin supplementation in ischemia/reperfusion-generated subjects, which clearly differentiated between I/R and Cur+I/R animals for both PPAR-α (P < 0.001) and PPAR-γ (P = 0.004), whereas no change of expression was observed in the Sham group (P > 0.05) (Figure 3A and B). Furthermore, PPAR-activated transcription factor of CREB1 was correspondingly decreased in the I/R group compared to controls (P = 0.002) and increased after using curcumin supplementation (P < 0.001), but no significant difference was detected between I/R and Sham groups, probably due to surgical stress (P > 0.05) (Figure 3C).
Relative mRNA Expressions of PPAR-α, PPAR-γ, and CREB1. Ischemia/reperfusion led to a decrease in PPAR-α, PPAR-γ, and CREB1 expressions. Curcumin supplementation upregulated the expressions of these transcription regulators. Asterisk (*) shows statistical significance (P < 0.05; Kruskal-Wallis and post-hoc Dunn’s tests) vs Control, section sign (§) vs Sham, and number sign (#) vs Cur+I/R. PPAR: Peroxisome proliferator-activated receptor. CREB1: cAMP responsive element binding protein-1.
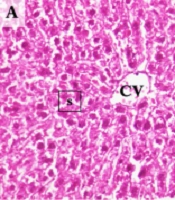
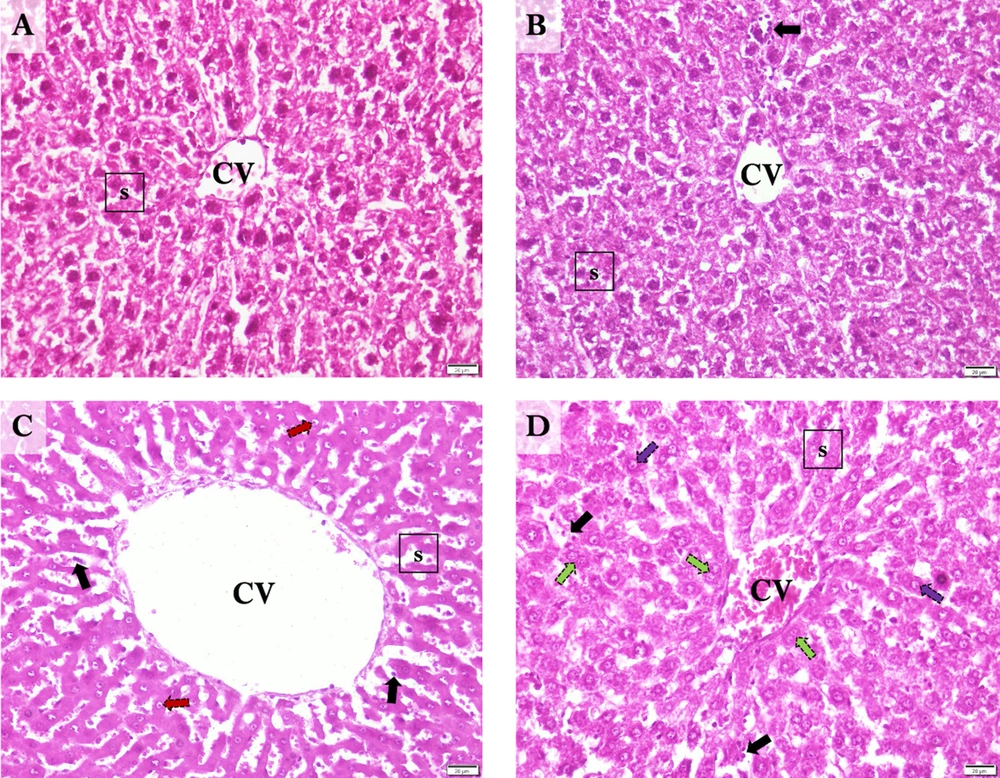
When examining the liver sections histologically, normal hepatic lobules with centrally nucleated healthy hepatocytes that organized radially arranged sinusoids reaching the central vein were observed in controls (Figure 4A). In the Sham group, normal liver histology was observed along with occasional sinusoidal lymphocytes (Figure 4B). The central vein and hepatic sinusoids were excessively dilated, and sinusoidal organization was disrupted in I/R samples. Also, an extensive occurrence of karyolitic hepatocytes was detected with occasional lymphocyte occupancy (Figure 4C). The hepatic microstructure was found to be, by and large, preserved in the ischemia/reperfusion-generated animals supplemented with curcumin. However, there was occasional nuclear vacuolization in hepatocytes accompanying to scarcely spread sinusoidal lymphocytes. Notably, numerous amounts of binucleated hepatocytes were marked, suggesting cell replication toward tissue regeneration (Figure 4D).
Representative Photomicrographs of the Liver Sections. A, Control; normal liver histology with visible central vein, hepatocytes, and sinusoids. B, Sham; normal central vein and sinusoids with occasional sinusoidal lymphocytes and cytoplasmic vacuolization in hepatocytes. C, I/R; Excessively dilated central vein, and dilated, disorganized sinusoids with karyolytic hepatocytes and occasional sinusoidal lymphocytes. D, Cur+I/R; Mildly dilated central vein and relatively protected sinusoidal organization with replicating hepatocytes alongside occasional nuclear vacuolization in hepatocytes and sinusoidal lymphocytes. CV: Central vein. s: Hepatic sinusoids. Black arrow: Sinusoidal lymphocytes. Red arrow: Karyolysis. Green arrow: Hepatocyte replication. Purple arrow: Nuclear vacuolization. Scale bar: 20 µm. Magnification: x400.
5. Discussion
In sum, the present study demonstrated that: (a) hepatic ischemia/reperfusion disrupted hepatic microstructure and downregulated PPAR-α, PPAR-γ, and CREB1 expressions; (b) curcumin supplementation in hepatic ischemia/reperfusion recovered structural organization while increasing PPAR-α, PPAR-γ, and CREB1 expressions; and (c) the crosstalk between targeted transcripts by ischemia/reperfusion and curcumin resulted in unaltered expressions of RelA and IκB.
Hepatic ischemia/reperfusion injury is a major problem, particularly in liver transplantations, that can exacerbate complications, including post-reperfusion syndrome, graft dysfunction or rejection, and chronic inflammation (34, 35). Considering the worldwide shortage of organ donations, attempts have been made to develop novel strategies aiming at improving graft survival rates and dealing with the loss of about one-fourth of liver transplants (36-40). To this end, it is necessary to explain the cellular mechanisms of both ischemia/reperfusion injury and potentially beneficial agents.
Curcumin is a well-defined antioxidant polyphenol showing strong anti-inflammatory activities that have been reported to improve hepatic ischemia/reperfusion injury (39). Since oxidative stress and inflammation feed each other, their molecular intersections (i.e., PPAR-α, PPAR-γ, CREB1, and the RelA/IκB complex) were hypothesized to be responsible for the injury as well as for the palliative property of curcumin. First, liver aminotransferases (along with bilirubin) were estimated and, as it was expected, were found to increase after hepatic ischemia/reperfusion. These enzymes reside in hepatocytes and are released in large amounts immediately when an injury occurs. Therefore, curcumin had no effect on serum aminotransferase concentrations, but the change in the enzyme levels biochemically confirmed the success of experimental modeling in both supplemented and non-supplemented animals in addition to the macroscopical and histochemical confirmation. The expressions of above-mentioned transcription factors were also investigated.
PPAR-α and PPAR-γ are mostly recognized for their roles in inflammation. The activation of toll-like receptors by cellular debris, altered plasma membrane, and/or reactive oxygen species initiates RelA signaling (40, 41) but, at the same time, activates PPAR-α and PPAR-γ that, in turn, inhibit RelA (42). Thereby, PPARs exert an anti-inflammatory effect in existence of a proinflammatory stimulus. In addition to the reciprocal relation between inflammation and oxidative stress, they are either stimulated or suppressed by reactive oxygen species alone, depending on the tissue type (43). In accordance with recent reports (44, 45) which have demonstrated an improvement with the activation of PPAR-α and PPAR-γ (and deterioration with inhibition) in hepatic ischemia/reperfusion, our study found that ischemia/reperfusion caused downregulation of PPAR-α and PPAR-γ. More importantly, curcumin supplementation resulted in increased expressions of PPARs. This finding was in line with result regarding the histological improvement and was suggestive of the contribution of PPAR activation to the hepatoprotective effect of curcumin. Even though only one study (46) had documented the curcumin-related activation of PPAR-γ in liver ischemia/reperfusion, our study provided supporting findings in this regard. To the best of our knowledge, moreover, our study is the first report that demonstrated the upregulation of PPAR-α in hepatic ischemia/reperfusion with curcumin supplementation.
Another target for inflammation and oxidative stress is the transcription factor of CREB1, which inhibits RelA and activates PPARs (20, 21). The PPAR activation is mediated by peroxisome proliferator-activated receptor gamma coactivator-1α (PGC-1α) (21, 47, 48), which also intercedes for the induction of synthesis of endogenous antioxidants to counteract oxidative stress (21), although Zhao et al. (49) reported that CREB1 attenuated glutathione synthesis and, therefore, weakened the antioxidant capacity. Confusingly, Pregi et al. (50) found an oxidative stress-induced upregulation in CREB1; however, they also reported an actuated DNA repair accompanying the increase of CREB1, which implies a time- and survivability-dependent expression pattern. Nevertheless, Özgen et al. (51) revealed a suppression in CREB1 expression with oxidative stress, which was supported by Motaghinejad et al.’s study (52), highlighting the downregulation in CREB1 expression with provoked inflammation and oxidative stress. Furthermore, the latter report also underscored the CREB1-inducing effect of curcumin. Therefore, our result regarding the upregulation of CREB1 by curcumin was consistent with the finding suggesting a structural improvement in the histological examination when there was a decreased CREB1 expression along with disrupted sinusoidal organization and apoptotic hepatocytes in non-supplemented ischemia/reperfusion samples. In our study, overall, it was suggested that the upregulation of CREB1 with curcumin supplementation may have contributed to the recovery of hepatic microstructure in hepatic ischemia/reperfusion.
Surprisingly, RelA and IκB expressions were found unaltered with ischemia/reperfusion or curcumin supplementation. Indeed, one of the most obvious ways through which ischemia/reperfusion triggers inflammation (and so, oxidative stress) is the liberation of RelA by degradation of IκB in the canonical pathway (53). Furthermore, the anti-inflammatory effect of curcumin is frequently attributed to the blockage of RelA activation (35, 54). However, deletion of RelA does not affect the inflammatory response for the first 1-2 days (55), and the prolonged oxidative stress inhibits RelA, whereas the activation of RelA is essential for cellular survival following the transient stress (56). As summarized above, ischemia/reperfusion-induced interactions are highly complex and, to some extent, compensative. The same stimulus that activates RelA also switches on opposing signals, including PPARs and CREB1. Moreover, RelA activity fluctuates in a time-dependent manner (57-59). Therefore, it was presumed that unaltered protein expressions of RelA and IκB in our study’s ischemia/reperfusion conditions were indicative of the intricate counterbalance.
5.1. Conclusions
Clarifying the mechanisms of ischemia/reperfusion injury and the pathways employed by potential hepatoprotective agents are extremely important for, particularly, liver transplantations. The present study revealed that hepatic ischemia/reperfusion modulated PPAR-α, PPAR-γ, and CREB1 expressions, and the curcumin supplementation protected the hepatic microstructure while upregulating the expressions of these transcription regulators.