1. Background
Osteoarthritis (OA) is a chronic, highly prevalent degenerative joint disease seen in half of people older than 65, which significantly affects their quality of life. Osteoarthritis is distinguished by the destruction or abrasion of articular cartilage, followed by secondary inflammation of synovial membranes, and leads to pain, stiffness, swelling, and disorders in joint articulation. Inflammatory factors, cellular and molecular mechanisms, and metabolic processes are involved in the incident and development of degenerative diseases, including OA (1, 2). Osteoarthritis affects many big and small joints, including knees, hips, spine, neck, fingers, thumb, and big toe. Age, sex, obesity, previous joint injury, heavy activities, high-impact sports, and genetics are the risk factors for osteoarthritis (3). In vivo preclinical animal models help study the mechanisms and pathogenesis of OA and evaluate new drug candidates.
Monosodium iodoacetate (MIA)-induced OA model in mice is rapid, widespread, and technically straightforward, with pathologic and pharmacologic characteristics close to human OA. Intra-articular injection of MIA causes synovial hyperplasia, penetrating inflammatory cells, destroying articular cartilage, disrupting chondrocyte metabolism, and producing cartilage degeneration and joint osteoarthritic (4). It has been shown that pain is a significant and the most undesirable symptom of OA, and painkiller medicines are essential in treating OA (5).
Nowadays, relieving pain and restoring the function of joints are the main goals of using pharmacologic therapies. Acetaminophen, naproxen, and other non-steroidal anti-inflammatory drugs (NSAIDs) and COX-2 inhibitors are comprehensively prescribed to relieve pain. The long-term use of these agents leads to undesirable effects including suppression of platelet aggregation, disruption of the gastrointestinal tract, and dysfunction of the kidneys and cardiovascular system. In recent years, the introduction of new therapeutic agents with good efficacy and low side effects has drawn a lot of attention (6).
Many people are interested in healthy foods and natural products, and many natural products and herbal medicines are efficient in pain relief. Since immemorial, plants, natural products, and herbal medicines have been particularly valuable and used for therapeutic goals. Ziziphus jujuba Mill. (Z. jujuba) as an herbal drug, is used in traditional medicine. Ziziphus jujuba is also known as red date or Chinese date and is called in the Persian pantry "annab." China is the center of origin of Z. jujuba production in the world. It is also distributed widely in other countries, especially tropical, subtropical, and Mediterranean regions (7, 8). Phytochemistry research indicated that Z. jujuba contains carbohydrates, minerals, vitamins, sugar, and amino acids. Consequently, this is a highly nutritious and functional food worldwide. Also, Z. jujuba is a rich source of flavonoids, polysaccharides, polyphenols, terpenoids, saponins, nucleotides, and others (9, 10). Based on the literature, bioactive compounds extracted from Z. jujuba have miscellaneous activities such as therapeutic effects on the nervous system, cardiovascular system, and gastrointestinal tract, improving the quality of sleep, as well as antioxidant, anti-inflammatory, and anti-cancer properties, and strong effects on blood purification (11). Furthermore, Z. jujuba can treat typhoid fever, diabetes, diarrhea, skin infections, anxiety, obesity, pharyngitis, bronchitis, anemia, and insomnia (12, 13).
In ancient times, natural resins had a significant role in Hindu, Babylonian, and Persian cultures. One of the high-quality resin-producing plants is Boswellia serrata Roxb., known as frankincense or olibanum. The species of Boswellia are widely distributed in India, Arabian Peninsula, North Africa, Somalia, Ethiopia, the Middle East, and Europe (14, 15). Traditionally, the resin of Boswellia serrata was used in many societies for the treatment of inflammatory diseases such as rheumatism, Crohn's disease, and ulcerative colitis. Previous studies reported some activities of Boswellia species such as anti-cancer, anti-inflammatory, immunomodulatory, antimicrobial, anti-viral, anti-atherosclerosis, analgesic, and antidiabetic activities. The phytochemical content of the resinous part of Boswellia serrata includes 30 - 60% terpenes, 5 - 10% essential oils, and polysaccharides. In vivo and in vitro studies showed that Boswellic acid inhibits the synthesis of pro-inflammatory enzymes, especially 5-lipoxygenase (5- LO). Inhibition of 5-lipoxygenase (5-LO) by boswellic acid leads to immune system suppression, decreased interleukins and TNF-α levels, diminished complement system, and reduced ROS formation (15, 16).
2. Objectives
The present study aimed to investigate the anti-nociceptive and anti-inflammatory activities of oral administration of hydroalcoholic extracts of Ziziphus jujuba (ZJE), Boswellia serrata (BSE), and both, using behavioral tests such as open-filed, foot-print, von-Frey, and hotplate tests. Biochemical studies were also done to measure IL-1β, IL-6, and TNF-α cytokines in the MIA-induced OA mice model.
3. Methods
3.1. Animals and Treatments
In this study, the standardized hydroalcoholic extract of Ziziphus jujuba fruits containing 1.0 % kaempferol and hydroalcoholic extract of Boswellia serrata gum containing 10.0% Boswellic acid were purchased from Morvariddasht Joven Cooperative Company, Iran. Seventy-two male NMRI mice (20 - 25 g) for all behavioral experiments and 12 adult female NMRI mice (25 - 30 g) for the acute toxicity test were obtained from the Animal House of School of Pharmacy, Shahid Beheshti University of Medical Sciences, Tehran, Iran. These animals were held in groups of eight per cage under standard animal laboratory conditions (25 ± 2°C temperature, 50 ± 5% humidity, and 12/12 h light/dark cycle with food and water ad libitum). All the experiments were organized attentively based on a protocol approved by the Ethics Committee of Shahid Beheshti University of Medical Sciences with approval code IR.SBMU.PHARMACY.REC.1401.040.
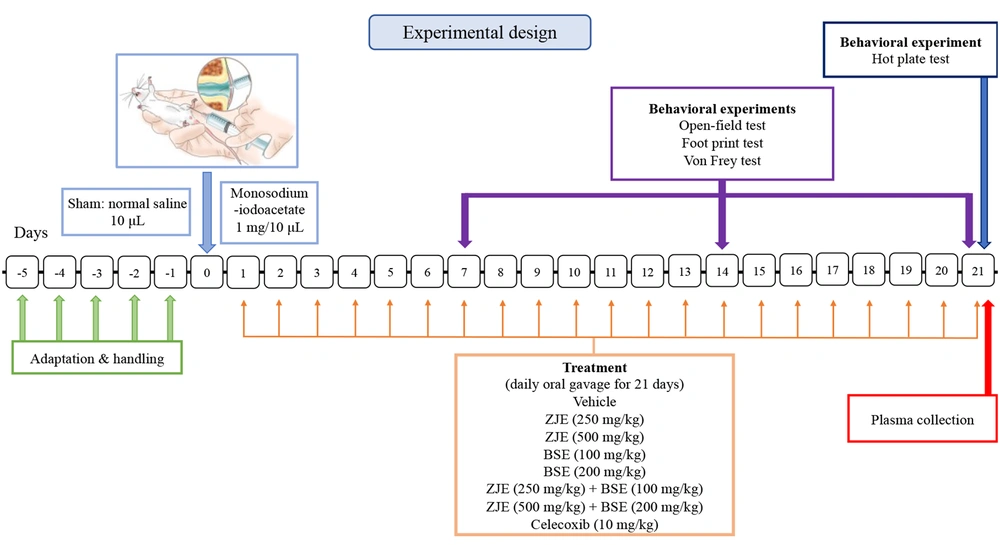
The animals were randomly divided into nine groups: (1) A sham group that received normal saline instead of MIA and eight MIA-induced osteoarthritis groups treated by (2) vehicle, (3) ZJE (250 mg/kg), (4) ZJE (500 mg/kg), (5) BSE (100 mg/kg), (6) BSE (200 mg/kg), (7) ZJE (250 mg/kg) + BSE (100 mg/kg), (8) ZJE (500 mg/kg) + BSE (200 mg/kg) extracts, and (9) Celecoxib (10 mg/kg; as positive control). MIA-induced osteoarthritis (OA) was conducted in mice according to the well-described methods of Ogbonna et al. and Pitcher et al. (17, 18). Daily oral administration was carried out for 21 days for the treatment groups starting from the day of OA induction by MIA. The experimental design and the time of each behavioral test are presented in Figure 1.
3.2. Behavioral Experiments
3.2.1. Open-field Test
The locomotor activity of the subjects was assessed by an open-field test as described previously by Jahani et al. (19). The total distance movement and velocity were analyzed by a tracking software program (Ethovision, Noldus, the Netherlands).
3.2.2. Foot-print test (Gait analysis test)
The walking tracks of the animals to determine their paw print area were evaluated using the foot-print test described by Del Barco et al. Then, ImageJ software was used for the analysis of the paw print areas (20).
3.3. Von Frey Test (Mechanical Sensitivity)
The mechanical allodynia of the hind paws was evaluated using von Frey filaments (North Coast Medical, Inc. CA, USA) in the von Frey test, which was a modified version of the Ziaei et al. method. The response to increasing pressure applied by von Frey filaments was measured as the paw withdrawal threshold (21).
3.4. Hot Plate Test (Thermal Hypersensitivity)
The hot plate test with a routine protocol is widely used to investigate anti-nociceptive activity (22). The latency of the withdrawal response of hind paws was determined by a hot plate test 21 days after injection of MIA.
3.5. Enzyme-linked Immunosorbent Assay
Enzyme-linked Immunosorbent Assay (ELISA) kit (Sigma, Saint Louis, USA) measured TNF-α, IL-6, and IL-1β to investigate whether these extracts affect inflammatory cytokines production. The colorimetric intensity was quantitated with a spectrophotometer (PerkinElmer, USA). Data were demonstrated as absorbance detected at 450 nm.
3.6. Acute Oral Toxicity (LD50 Determination)
Acute toxicity is described as the unwanted effects that occur immediately or at a short time interval after single or multiple administrations of each substance within 24 hours. For assessing the lethal dose (LD50), Lorke's method was used. The results of the study were used to determine the appropriate toxicity classification. Classification of LD50 based on dose range was shown by Chinedu et al. and Erhirhie et al. (23, 24).
3.7. Statistical Analysis
All the data were represented as mean ± standard error of the mean (SEM) and analyzed by one-way and two-way analysis of variance. Then, Tukey's and Bonferroni's post hoc tests were used when appropriate to investigate the differences between the groups at a significance level of P < 0.05. Image J software was used to measure the pixel values in the foot-print test. All the analyses were carried out by the GraphPad Prism (v. 8) software.
4. Results
4.1. Body Weight Changes
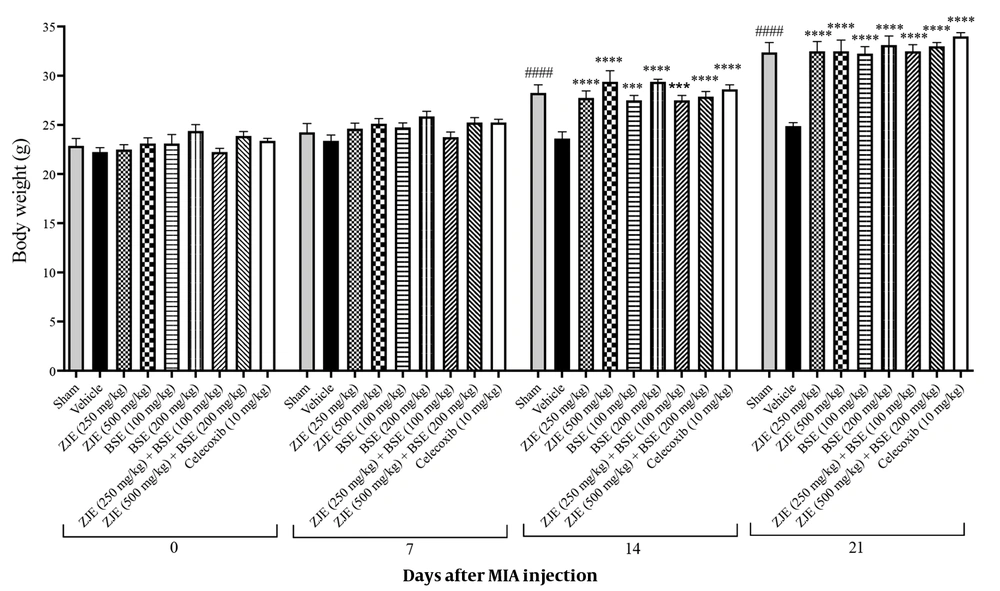
The body weight of each animal was monitored every day during the 21 days of the experiment. Figure 2 shows the body weight of mice in all groups at 0, 7, 14, and 21 days after injection of MIA. Treatment with ZJE (250 and 500 mg/kg), BSE (100 and 200 mg/kg), ZJE (250 mg/kg) + BSE (100 mg/kg), and ZJE (500 mg/kg) + BSE (200 mg/kg) significantly increased the body weight compared to the vehicle group after two weeks (effect of time F(3, 189) = 922.5, P < 0.0001, effect of treatment F(8, 63) = 5.9, P < 0.0001, effect of interaction F(24, 189) = 8.9, P < 0.0001).
Effects of treatments with Ziziphus jujuba extract (ZJE), Boswellia serrata extract (BSE), and celecoxib (positive control) on body weight. Data were collected over 21 consecutive days after the injection of MIA. The results were analyzed by two-way ANOVA, followed by Bonferroni's test. Data are described as mean ± SEM (n = 8). ***P < 0.001 and ****P < 0.0001 compared to the vehicle group. ####P < 0.0001 compared the sham group with the vehicle group.
4.2. Open Field Test
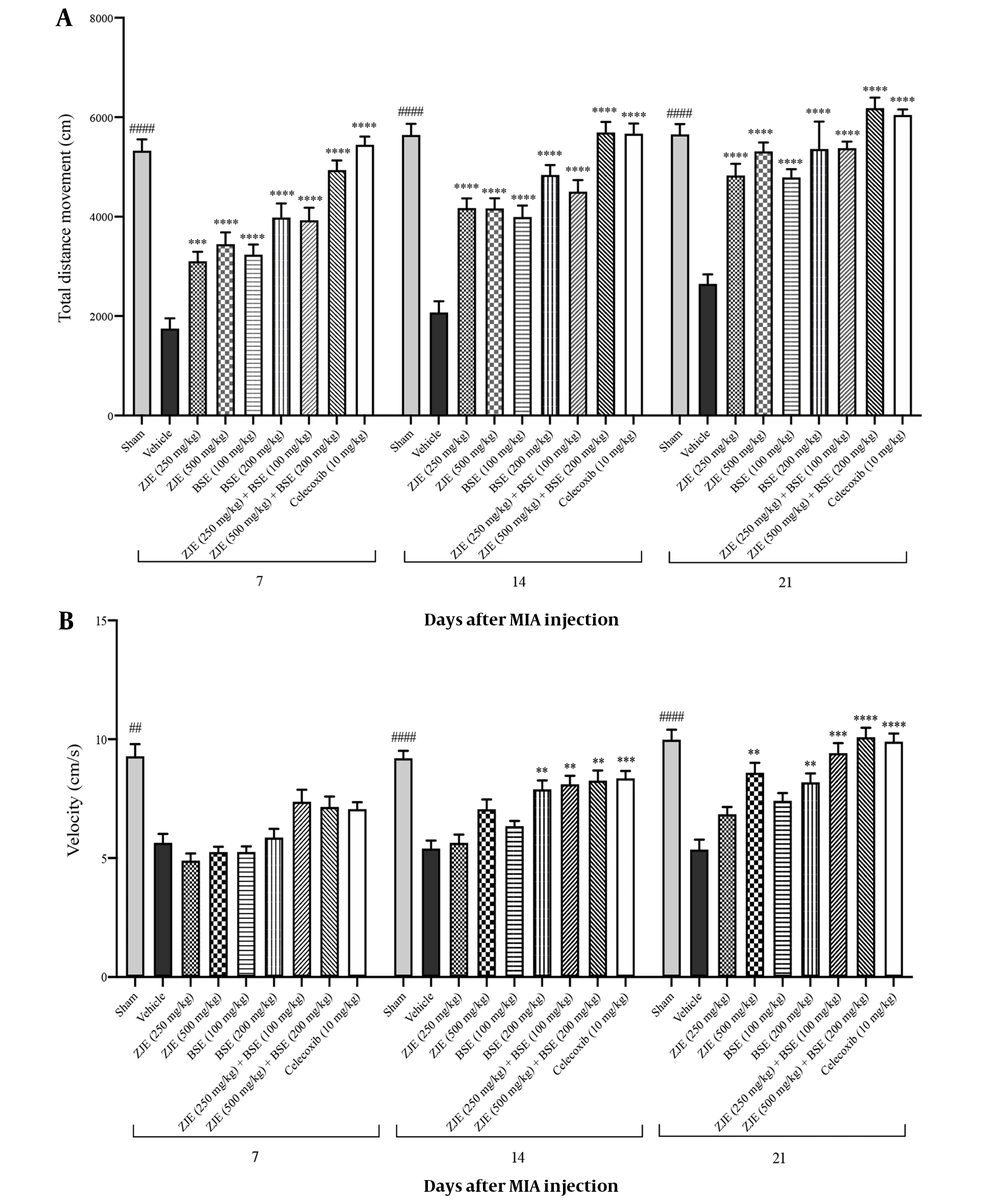
Total distance movement and velocity during 10 min showed significant differences after 7, 14, and 21 days compared to the vehicle group. As shown in Figure 3A, ZJE at doses of 250 mg/kg and 500 mg/kg after 7, 14, and 21 days significantly increased the total distance moved by the animals compared to the vehicle group. BSE at doses of 100 mg/kg and 200 mg/kg after 7, 14, and 21 days significantly increased the total distance moved by animals compared to the vehicle group. ZJE (250 mg/kg) + BSE (100 mg/kg) after 7, 14, and 21 days significantly increased the total distance moved by the animals compared to the vehicle group. ZJE (500 mg/kg) + BSE (200 mg/kg) after 7, 14, and 21 days significantly increased the total distance moved by the animals compared to the vehicle group (effect of time F(2, 126) = 60.2, P < 0.0001, effect of treatment F(8, 63) = 80.3, P < 0.0001, effect of interaction F(16, 126) = 1.3, P =0.018).
As shown in Figure 3B, ZJE at a dose of 500 mg/kg after 21 days significantly increased the velocity of animals compared to the vehicle group. BSE at a dose of 200 mg/kg after 14 and 21 days significantly increased the velocity of animals compared to the vehicle group. ZJE (250 mg/kg) + BSE (100 mg/kg) after 14 and 21 days significantly increased the velocity of animals compared to the vehicle group. ZJE (500 mg/kg) + BSE (200 mg/kg) after 14 and 21 days significantly increased the velocity of animals compared to the vehicle group (effect of time F(1.8, 114.3) = 93.8, P < 0.0001, effect of treatment F(8, 63) = 26.2, P < 0.0001, effect of interaction F(16, 126) = 3.9, P < 0.0001).
Effects of treatments with Ziziphus jujuba extract (ZJE), Boswellia serrata extract (BSE), and Celecoxib (positive control) on total distance movement (A) and velocity (B) in the open field test. Data were collected over three consecutive weeks (7, 14, and 21 days after injection of MIA). The results were analyzed by two-way ANOVA, followed by Bonferroni's test. Data are described as mean ± SEM (n = 8). **P < 0.01, ***P < 0.001, and ****P < 0.0001 compared to the vehicle group. ##P < 0.01 and ####P < 0.0001 compared the sham group with the vehicle group.
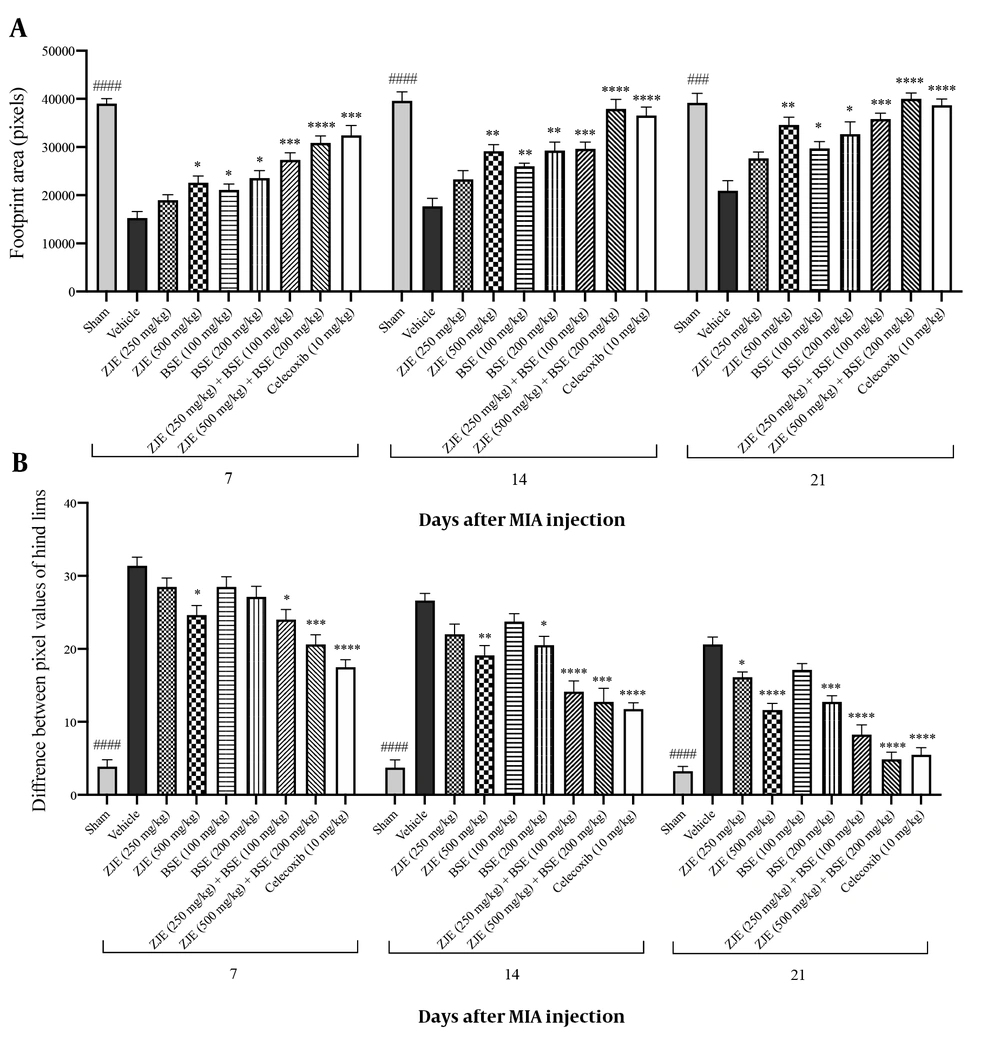
4.3. Foot-print Test
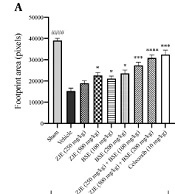
Images of foot-print patterns showed the abnormalities in the foot placement. Animals loaded their weights in the medial part of their feet. As shown in Figure 4A, ZJE at a dose of 500 mg/kg after 7, 14, and 21 days significantly increased the pixel values of the foot-print area compared to the vehicle group. BSE at a dose of 100 mg/kg after 7, 14, and 21 days significantly increased the pixel values of the foot-print area compared to the vehicle group. BSE at a dose of 200 mg/kg after 7, 14, and 21 days significantly increased the pixel values of the foot-print area compared to the vehicle group. ZJE (250 mg/kg) + BSE (100 mg/kg) after 7, 14, and 21 days significantly increased the pixel values of the foot-print area compared to the vehicle group. ZJE (500 mg/kg) + BSE (200 mg/kg) after 7, 14, and 21 days significantly increased the pixel values of the foot-print area compared to the vehicle group (effect of time F(1.9, 123.6) = 58.6, P < 0.0001, effect of treatment F(8, 63) = 45.4, P < 0.0001, effect of interaction F(16, 126) = 1.4, P = 0.124). Additionally, celecoxib at a dose of 10 mg/kg after 7, 14, and 21 days significantly increased the pixel values of the foot-print area compared to the vehicle group (P < 0.001, P < 0.0001, and P < 0.0001, respectively). As shown in Figure 4B, ZJE at a dose of 250 mg/kg after 21 days significantly decreased the difference between the pixel values of hind limbs compared to the vehicle group. ZJE at a dose of 500 mg/kg after 7, 14, and 21 days significantly decreased the difference between the pixel values of hind limbs compared to the vehicle group. BSE at a dose of 200 mg/kg after 14 and 21 days significantly decreased the difference between the pixel values of hind limbs compared to the vehicle group. ZJE (250 mg/kg) + BSE (100 mg/kg) after 7, 14, and 21 days significantly decreased the difference between the pixel values of hind limbs compared to the vehicle group. ZJE (500 mg/kg) + BSE (200 mg/kg) after 7, 14, and 21 days significantly decreased the difference between the pixel values of hind limbs compared to the vehicle group (effect of time F(1.7, 112.8) = 398.9, P < 0.0001, effect of treatment F(8, 63) = 57.9, P < 0.0001, effect of interaction F(16, 126) = 6.9, P < 0.0001). Additionally, celecoxib at a dose of 10 mg/kg after 7, 14, and 21 days significantly decreased the difference between the pixel values of hind limbs compared to the vehicle group (P < 0.0001, P < 0.0001, and P < 0.0001, respectively).
Effects of treatment with Ziziphus jujuba extract (ZJE), Boswellia serrata extract (BSE), and Celecoxib (positive control) on pixel values of foot-print area (A) and the difference between pixel values of hind limbs (B) in the foot-print test. Data were collected over three consecutive weeks (7, 14, and 21 days after injection of MIA). The results were analyzed by two-way ANOVA, followed by Bonferroni's test. Data are described as mean ± SEM (n = 8). *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001 compared to the vehicle group. ###P < 0.001 and ####P < 0.0001 compared the sham group with the vehicle group.
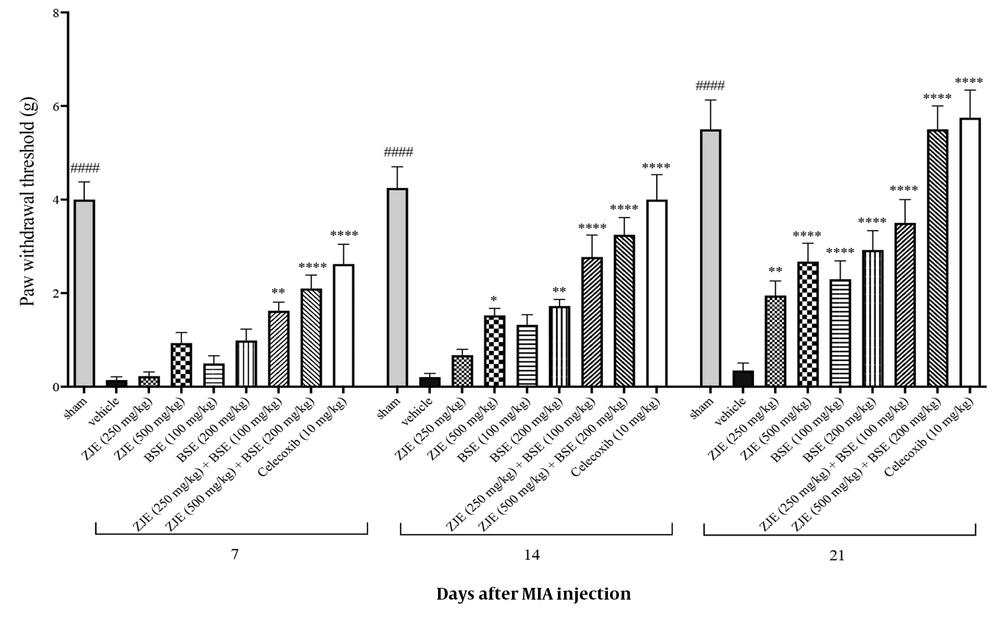
4.4. Von Frey Test (Mechanical Sensitivity)
As shown in Figure 5, ZJE at a dose of 250 mg/kg after 21 days significantly increased the pain threshold compared to the vehicle group. ZJE at a dose of 500 mg/kg after 14 and 21 days significantly increased the pain threshold compared to the vehicle group. BSE at a dose of 100 mg/kg after 21 days significantly increased the pain threshold compared to the vehicle group. BSE at a dose of 200 mg/kg after 14 and 21 days significantly increased the pain threshold compared to the vehicle group. ZJE (250 mg/kg) + BSE (100 mg/kg) after 7, 14, and 21 days significantly increased the pain threshold compared to the vehicle group. ZJE (500 mg/kg) + BSE (200 mg/kg) after 7, 14, and 21 days significantly increased the pain threshold compared to the vehicle group (effect of time F(2, 14) = 148.2, P < 0.0001, effect of treatment F(8, 56) = 26.7, P < 0.0001, effect of interaction F(16, 112) = 3.4, P < 0.0001).
Effects of treatment with Ziziphus jujuba extract (ZJE), Boswellia serrata extract (BSE), and Celecoxib (positive control) on paw withdrawal threshold in von Frey test. Data were collected over three consecutive weeks (7, 14, and 21 days after injection of MIA). The results were analyzed by two-way ANOVA, followed by Bonferroni's test. Data are described as mean ± SEM (n = 8). *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001 compared to the vehicle group. ####P< 0.0001 compared the sham group with the vehicle group.
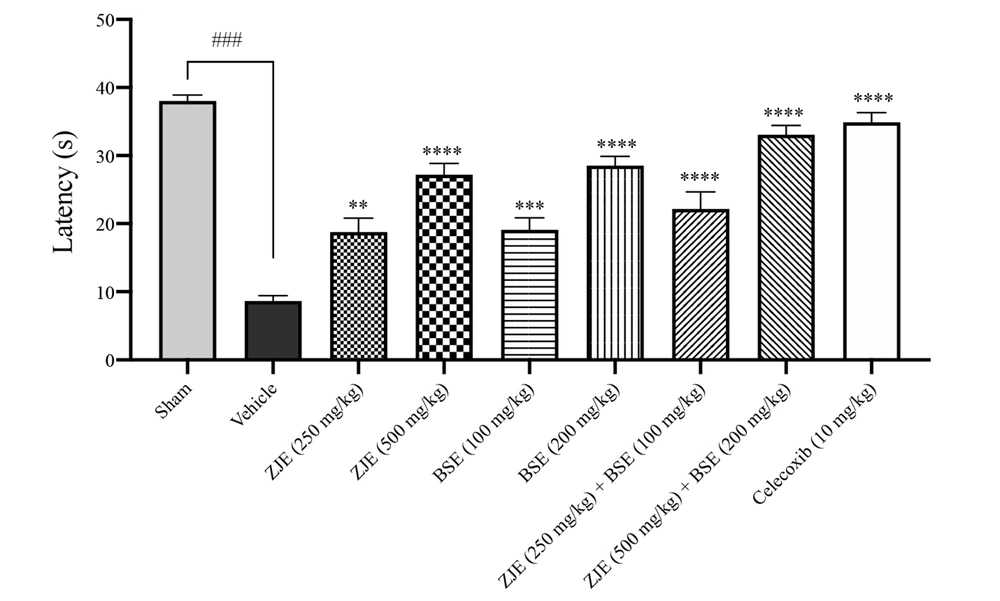
4.5. Hot Plate Test (Thermal Hypersensitivity)
As shown in Figure 6, ZJE (250 and 500 mg/kg), BSE (100 and 200 mg/kg), ZJE (250 mg/kg) + BSE (100 mg/kg), ZJE (500 mg/kg) + BSE (200 mg/kg), and celecoxib (10 mg/kg) after 21 days significantly increased the latency of the withdrawal response to heat stimulation compared to the vehicle group (P < 0.01, P < 0.0001, P < 0.001, P < 0.0001, P < 0.0001, P < 0.0001, and P < 0.0001, respectively).
Effects of treatment with Ziziphus jujuba extract (ZJE), Boswellia serrata extract (BSE), and Celecoxib (positive control) on the latency of the withdrawal response to heat stimulation in hot plate test. Data were collected 21 days after the injection of MIA. The results were analyzed by one-way ANOVA, followed by Tukey's test. Data are described as mean ± SEM (n = 8). **P < 0.01, ***P < 0.001, and ****P < 0.0001 compared to the vehicle group. ###P< 0.001 compared the sham group with the vehicle group.
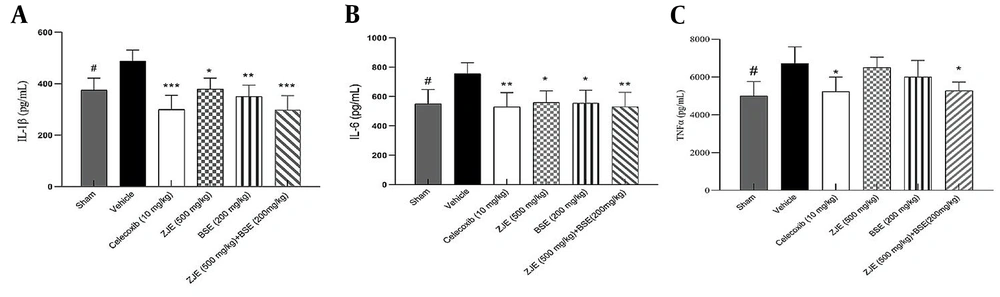
4.6. Effect of ZJE and BSE on IL-1β, IL-6, and TNF-α Pro-inflammatory Cytokines
The effects of ZJE and BSE on the levels of IL-1β, IL-6, and TNF-α inflammatory cytokines were measured with ELISA. Figure 7 shows that the cytokine release was considerably lower in ZJE (500 mg/kg), BSE (200 mg/kg), and ZJE (500 mg/kg) + BSE (200 mg/kg) groups than in the vehicle group. Also, celecoxib as positive control significantly suppressed cytokine production.
Effect of treatment with Ziziphus jujube extract (ZJE), Boswellia serrata extract (BSE), and celecoxib (positive control) on the production of (a) IL-1β, (b) IL-6, and (c) TNF-α cytokines. The results were analyzed by one-way ANOVA, followed by Tukey's test. Data are described as mean ± SEM. *P < 0.05, **P < 0.01, and ***P < 0.001 compared to vehicle group. #P < 0.05 and ##P < 0.01 compared the sham group with the vehicle group.
4.7. Acute Oral Toxicity (LD50 Determination)
At the end of the study, the acute toxicity showed no mortality in both phases of the acute oral toxicity test. Under the conditions of this study, ZJE and BSE produced no mortality in adult female mice. The LD50 of ZJE and BSE was greater than 5,000 mg/kg.
5. Discussion
Osteoarthritis (OA) is a degenerative arthropathy in articular cartilage that induces problems in inflammatory pathways and leads to pain. Production of inflammatory cytokines such as interleukin-1b (IL-1b), interleukin-6 (IL-6), and tumor necrosis factor (TNF)-a in articular cartilage causes degradation and apoptosis of chondrocytes and increases cartilage degeneration and damage (25, 26). Numerous drugs and medicines have been used to treat OA, but these agents have numerous side effects. Meanwhile, the usage of traditional and natural products with the minimum side effects has been considered by physicians, researchers, and patients (27). In this study, we investigated the anti-nociceptive and anti-inflammatory effects of hydroalcoholic extracts of Ziziphus jujuba, Boswellia serrata, and their combination using open-filed, foot-print, von Frey, and hotplate tests and measuring IL-1β, IL-6, and TNF-α cytokines in the MIA-induced OA mice model compared to celecoxib as the positive control.
In this study, the body weights were reported weekly. All animals were weighed daily during the experimental period. Mice in the sham and treatment groups had normal food intake and gained similar body weight. But the vehicle group gained less body weight than the other groups during the experiment. These results showed that ZJE (250 and 500 mg/kg), BSE (100 and 200 mg/kg), and celecoxib (10 mg/kg) had noticeable effects on body weight after two weeks in mice. Numerous factors should be considered for the explanation of these changes. The corticotropin-releasing factor (a potent anorexic agent) may increase during pain, inflammation, and stress conditions (28). Ou et al. indicated that the group of animals with the joint disorder compared to the control group showed significantly increased serum corticosterone levels in the hippocampus (29). The secretion of the corticotropin-releasing hormone by the hypothalamus activates the anterior pituitary to secrete the adrenocorticotropic hormone (ACTH). ACTH increases the synthesis of cortisol and other glucocorticoids. Otherwise, it is most likely that the animals that suffer pain (without treatment) have less motor activity, energy, and effort to obtain food (30).
According to the open-field test, the oral administration of all extracts significantly increased the total distance movement compared to the vehicle group during the 21 days of the experiment. The oral co-administration of ZJE + BSE and BSE (200 mg/kg) alone significantly increased the velocity of animals compared to the vehicle group after two weeks during the 21 days of the experiment. The open field test is one of the objective measures of pain-related behavior and anti-nociceptive activity of compounds in rodents. Intra-articular injection of MIA in the rat left knee joint produces a significant reduction in locomotor activity (31). Oh et al. reported that ethanol extract of Ziziphus jujuba Mill var. Spinosa seeds exhibited significant anti-depressant-like effects by increasing the mobility time and total distance in the force swimming and open field tests (32). In previous studies, pretreatment with an aqueous extract of B. serrata enhances the rats' performances in the Morris water maze and open field tests and corrects memory impairment in LPS-induced inflammation (33). Similarly, boswellic acids (BAs) and pentacyclic triterpene molecules enhance locomotor performance, reduce inflammatory factors, and increase striatal dopamine levels in rats with rotenone-induced parkinsonism (34).
Based on the results of the foot-print test, the oral administration of extracts (in all the treatment groups except for ZJE at 250 mg/kg) significantly increased the pixel values of the foot-print area compared to the vehicle group during the 21 days of the experiment. The oral co-administration of ZJE + BSE and ZJE (500 mg/kg) alone after the first week and BSE (200 mg/kg) alone after the second week significantly decreased the difference between pixel values of hind limbs compared to the vehicle group during 21 days of the experiment. Animal gait patterns and foot-print areas may be reduced in inflammatory joint diseases such as osteoarthritis. Furthermore, the topical application of many plants like Lawsonia inermis L. and Ricinus communis L. improves the foot-print areas and gait patterns in knee osteoarthritis (21). The literature review discovers that the anti-inflammatory and anti-arthritic properties of Boswellia serrata are attributed to β-boswellic acid, acetyl-β-boswellic acid, 11-keto-β-boswellic acid, and acetyl-11-keto-β-boswellic acid (14).
In the von Frey test, the oral co-administration of ZJE + BSE after the first week and ZJE (500 mg/kg) and BSE (200 mg/kg) after the second week significantly increased the paw withdrawal threshold compared to the vehicle group during 21 days of the experiment. An earlier publication by Alsalem et al. indicated that MIA causes a significant reduction in the paw withdrawal threshold (31). A recent study showed that after 21 days, MIA induced mechanical allodynia and decreased the expression of spinal cord sirtuin 1, which plays a critical role in continuous pain (35). Natural flavanol, such as kaempferol, found in herbal medicine, provides antioxidant and neuroprotective activities; it can increase the threshold of withdrawal latency in mechanical allodynia (36). Also, the evidence indicates that boswellic acid alleviates thermal hypersensitivity and mechanical allodynia in chronic constriction diseases and neuropathic pain (37, 38).
According to the hot plate test results, the oral administration of the extract in all treatment groups significantly increased the latency of withdrawal response to heat stimulation compared to the vehicle group after 21 days of the experiment. Nowadays, gene therapy and platelet-rich plasma (PRP) are protected against thermal hyperalgesia using a hot plate test in mature mice (39). Traditional treatments are interesting besides these novel techniques. The study of Kandimalla et al. explained the neuroprotective activity of bioactive components of Annona reticulata and Ziziphus jujuba, which potentially attenuated the thermal and mechanical hyperalgesia and cold allodynia better than insulin (40). Hot plate and writhing reflex techniques assessed the central and peripheral analgesic activities of nine kaempferol glycoside and acyl derivatives. These agents protect animals against pain, but the induction of peripheral analgesia is stronger than the central effect (41). Additionally, numerous fractions of Boswellia serrata showed analgesic activity by acetic acid-induced writhing reflex, formalin-induced pain, hot plate, and tail-flick techniques (42).
Our results of in vitro studies showed that the levels of IL-1β, IL-6, and TNF-α were reversed by ZJE (500 mg/kg), BSE (200 mg/kg), and ZJE (500 mg/kg) + BSE (200 mg/kg) treatments, similar to celecoxib (10 mg/kg). These results suggest that IL-1β, IL-6, and TNF-α play a significant role in the rheumatoid arthritis inflammatory process, and their release could be controlled with an effective treatment such as Celecoxib. Each of the extracts alone significantly inhibited IL-1β and IL-6, while they had no substantial influence on TNF-α. However, the co-administration of ZJE+BSE inhibited all three cytokines and demonstrated a more significant effect than taking each alone. These results indicate that the potency of ZJE, BSE, and their co-administration is not less than that of celecoxib. Further studies showed that pro-inflammatory cytokines, such as IL-1β, IL-6, and TNF-α, are the primary mediators in OA pathophysiology (43). Malemud, in 2010, indicated that IL-1, IL-6, and TNF-α play prominent roles in the development of articular cartilage extracellular matrix protein degradation (44). Based on the evidence, the oral administration of Ziziphus Jujuba cv. Pozao to cyclophosphamide-induced ICR mice for 28 days significantly improved lymphocyte proliferation, decreased the proportion of CD3+ and CD4+, increased the levels of IL-2, IL-4, IL-10, IFN-γ, and TNF-α in serum, and increased the levels of IgA (45).
Also, Z. jujuba methanol extract at 200 and 400 mg/kg and Z. jujuba water fraction at 50 and 100 mg/kg inhibited the lipid peroxidation and significantly repaired the cytokine levels such as TNF-α, Il-1β, and Il-10 against hepatic injury and chronic inflammation induced by CCl4 (46). Extracts of Boswellia serrata and its ingredients, including Boswellic acid, affect the immune system by releasing cytokines. BAs inhibit the activation of nuclear factor-κB (NF-κB), down-regulate TNF-alpha, and reduce IL-1, IL-2, IL-4, IL-6, and IFN-gamma (47). In Kim et al. study, LI73014F2 (a herbal composition including Terminalia chebula, Curcuma longa, and Boswellia serrata) was orally administered in monosodium iodoacetate (MIA)-induced osteoarthritis (OA). LI73014F2 significantly inhibited the cyclooxygenase-2 (COX-2) and 5-lipoxygenase (5-LOX) activities, reduced IL-1β, and inhibited the NF-κB/MAPK signaling pathway (48). According to the acute oral toxicity test in mice, ZJE and BSE are practically nontoxic and have a high degree of safety in the condition of this study. Dodda et al. determined that the median lethal dose of Boswellia serrata gum resin extracts was above 2,000 mg/kg in the acute oral and dermal toxicity studies in Sprague Dawley (SD) rats (49).
5.1. Conclusions
The oral administration of ZJE and BSE presented anti-nociceptive and anti-inflammatory properties, significantly reduced OA disease progression, and showed an analgesic effect. This study is the first report on the co-administration of ZJE and BSE in treating the monosodium iodoacetate (MIA)-induced osteoarthritis model in mice. We believe that the chronic oral co-administration of ZJE (500 mg/kg) and BSE (200 mg/kg) extracts can be used as appropriate herbal medicine to antagonize the development of OA, possibly as a suitable replacement for celecoxib during OA progress.