1. Background
Ruptured vertebral artery dissecting aneurysms (VADAs) are well-known for a high risk of recurrent bleeding and high mortality rates (1, 2). Furthermore, most recurrent bleeding (ranging from 57% to 93%) occurs within 24 hours of the first hemorrhagic event, emphasizing the necessity of early and aggressive treatment (3, 4). Under these emergent circumstances, surgical interventions carry a relatively high risk of treatment-related morbidity and mortality (5, 6). In recent years, endovascular treatment has been the preferred first-line treatment method for ruptured VADA, and various endovascular techniques, including newer parent artery preservation techniques, have been introduced as possible treatment methods for this challenging condition (7-9). However, the efficacy and long-term follow-up results of the various endovascular treatments have not been well established.
Treatment strategies for a ruptured VADA should be individualized according to the severity of the presenting symptoms, the location of the dissected aneurysm in the parent artery with respect to the major perforating branches, and the adequacy of collateral supplies (4, 10-12). Therefore, appropriate angiographic analysis of a ruptured VADA is an essential starting point from which to determine the optimal endovascular treatment method. In this study, we performed a specifically selected mode of endovascular treatment for patients with ruptured VADAs, based on each patient’s clinical condition and the angiographic features of the dissected aneurysm. In addition, we monitored patients undergoing endovascular treatment over a period of one year, and assessed the clinical, angiographic, and procedural predictors of clinical outcomes.
2. Objectives
The purpose of this retrospective study was to report the therapeutic efficacy, long-term imaging, and clinical follow-up results of various endovascular techniques that were performed on the basis of angiographic subtypes of ruptured VADAs, as well as the predictors of clinical outcomes.
3. Patients and Methods
3.1. Patients
The institutional review boards of all participating hospitals approved this retrospective study and waived informed consent. Between January 2010 and August 2014, 30 patients with ruptured VADAs underwent endovascular treatment at the two participating institutions. All patients fulfilled the following inclusion criteria: 1) acute onset of subarachnoid hemorrhage (SAH) relevant to VADA, as confirmed by brain CT scan; 2) angiographic findings of VADA, such as pearl-and-string sign, double lumen, beaded appearance of the parent artery, or fusiform dilatation with delayed clearance of the dilated lumen; and 3) having undergone endovascular treatment for VADA. Patients who presented with clinical symptoms other than SAH, such as ischemic symptoms of posterior circulation, sudden onset of severe headache, or a combination of these, were excluded. Patients who had dissecting aneurysms that involved the basilar artery, or in whom dissecting aneurysms not corresponding to the clinical symptoms were incidentally found, were also excluded. A total of 20 males and 11 females were included, with a mean age of 47.0 years (range: 2 - 59 years). Each patient’s clinical condition on admission was assessed using the world federation of neurosurgical societies (WFNS) grading system; 12 cases were grade I - II, four were grade III, and 14 were grade IV - V. For ease of analysis, the initial WFNS grades were categorized as fair (WFNS grade I - III) or poor (WFNS grade IV - V) conditions. Treatment was initiated within 24 hours after SAH, with the exception of one patient who was transferred from another hospital; this patient was treated five days after initial symptom onset.
3.2. Angiographic Analyses
All patients enrolled in this study underwent transfemoral cerebral angiography to confirm diagnosis and to evaluate the precise angioarchitecture of the lesion. Angiographic analyses were carried out to determine the status of the following conditions: 1) whether the dissecting aneurysm involved the dominant vertebral artery (VA), 2) whether the dissecting aneurysm involved the orifice of major perforating vessels, and 3) whether collateral circulation via the contralateral posterior inferior cerebellar artery (PICA) or the ipsilateral anterior inferior cerebellar artery (AICA) was adequate. The dissecting aneurysms were classified into two main groups depending on whether the involved VA was dominant. Dominant VADA was defined as when the contralateral VA showed hypoplasia or when insufficient cerebral blood flow into the posterior circulation was expected if the involved VA was sacrificed. In contrast, non-dominant VADA had a well-developed contralateral VA or posterior communicating artery (PCoA), which guaranteed sufficient blood supply into the posterior circulation even after occluding the involved VA. Non-dominant VADAs were further subdivided into three types according to which major perforating vessels were involved, as follows: 1) PICA-involved type, 2) anterior spinal artery (ASA)-involved type, or 3) non-involved type, in which the dissected segment does not bear the orifice of the PICA nor of the ASA.
3.3. Endovascular Treatment Strategies and Treatment Outcomes
Endovascular treatments for ruptured VADAs consisted of two major techniques: a reconstructive technique, including stent-assisted coiling (SAC) and stent-only therapy (SOT) with single or multiple stents, and a deconstructive technique, including proximal occlusion of the parent artery and internal coil trapping (ICT). We adopted both SAC and SOT as reconstructive techniques for patients with a dominant VADA because these preserve the antegrade flow of the involved parent artery and perforating vessels. In patients with a non-dominant VADA involving the orifice of the PICA, either reconstructive or deconstructive endovascular treatment was performed. If the patient had adequate collateral supplies from the contralateral PICA or the ipsilateral AICA, ICT was performed. Internal trapping, which requires obliteration of the dissected aneurysm and the involved segment of the parent artery by coils, was the only deconstructive technique performed in this study, and was mainly used to treat the non-involved type. When the involved PICA was a dominant supplying artery to the cerebellum, SAC was performed at the most clearly dilated site to preserve the PICA origin. When treating a non-dominant VADA that involved an orifice of the ASA, SOT with multiple stents was performed to preserve the antegrade flow of the ASA. In most cases, the exact location of the orifice of the ASA was not delineated on either digital subtracted angiography (DSA) or three dimensional (3D)-reformatted angiography; thus, SAC was not used, for fear of incidental obliteration of the ASA origin.
Immediate angiographic results were classified into two categories, according to the degree of obliteration of the dissecting aneurysm: complete obliteration (the entire dilated aneurysm or dissected segments were obliterated) or partial obliteration (contrast-filling was visualized in the dissected or aneurysmal dilated segments). Procedure-related complications, such as in-stent occlusion, were recorded. Post-procedural complications, such as thromboembolic infarction delineated on post-procedural diffusion-weighted images (DWIs), were also recorded.
3.4. Follow-Up Protocols
The modalities for imaging follow-up were determined according to each individual’s treatment method. For patients managed with a reconstructive technique, follow-up DSA was performed 3 - 6 months and 1 - 2 years after the initial treatment. Follow-up magnetic resonance (MR) angiography was performed for patients treated with a deconstructive technique six months and one year after treatment. DSA follow-up was reserved for patients with suspicious recanalization of the obliterated parent artery on follow-up MR angiography. Imaging follow-up results were classified into two categories: 1) stable occlusion, in which complete obliteration of the dissecting aneurysm was maintained or when the residual sac showed no internal change in size and configuration, and 2) recurrence, in which rebleeding occurred or recanalization was demonstrated after complete obliteration, or the residual sac showed an internal size increase after partial obliteration. In-stent stenosis or occlusion, and patency of antegrade flow of the parent artery and perforating vessels covered by stents, were evaluated on follow-up imaging studies.
The clinical follow-up results were assessed with a modified Rankin scale (mRS) score during the follow-up period. The mRS score at one year after treatment was used to evaluate each patient’s clinical status. A score of 0 - 2 on the mRS, which indicates that the patient can live an independent life, was categorized as a favorable outcome, while a score of 3 - 6 one year after mRS was categorized as a poor outcome.
3.5. Statistical Analysis
Statistical analysis was performed using SPSS software ver. 22.0 (IBM Corp. Released 2013. IBM SPSS Statistics for Windows, Armonk, NY: IBM Corp.). Univariate statistical analysis was performed by using the χ2 or Fisher’s exact test to assess associations between clinical, angiographic, and procedural variables and long-term clinical follow-up results. Variables with P values of < 0.10 in the univariate analysis were chosen for multivariate models using a logistic regression analysis. Statistical significance was defined as P < 0.05 in all analyses, and the P values and 95% confidence interval (CI) for odds ratio (OR) were corrected by Bonferroni’s method for multiple tests.
4. Results
4.1. Angiographic Classification and Mode of Endovascular Treatment
The angiographic subtypes and applied treatment modalities are summarized in Figure 1. All five patients with dominant VADAs were treated with a reconstructive technique to preserve the antegrade flow of the involved VA. In the early phase of the study, SAC, with either a single stent (n = 1) or double stents (n = 2), was used, but SOT with triple stents (n = 2) was used more frequently in the late phase of the study.
SOT with triple stents (n = 3) was used for PICA-involved cases, and SOT with double stents (n = 1) was used for the ASA-involved case. SAC with a single stent (n = 2) or double stents (n = 2) was used in patients with PICA-involved lesions, to preserve the antegrade flow of the PICA. One patient with a non-dominant VADA that involved a dominant PICA underwent modified ICT to preserve the orifice of the involved PICA, and, in this case, a small remnant was intentionally left and was classified as a partial obliteration. The remaining 16 internal trapping procedures were performed by obliterating both the involved parent artery and the perforating vessels.
4.2. Immediate Angiographic Results and Follow-Up Treatment Outcomes
Immediate angiographic results and follow-up treatment outcomes are summarized in Table 1. Follow-up images, which were available for 27 patients, were taken at 6 - 62 months (mean, 16 months) after treatment, except for three patients who expired during hospitalization. Of the six patients classified as having had a partial obliteration, three had recurrent hemorrhage during hospitalization, and two were retreated for enlarged residual sacs with follow-up angiography. Two of the three patients who had rebleeding episodes died as a consequence. All 20 of the surviving patients who had complete obliteration showed durable occlusion of the dissected aneurysm on follow-up imaging studies. Clinical follow-up was available in all 25 surviving patients at 12 - 69 months (mean, 27.5 months) after treatment, and favorable clinical outcomes were achieved in 17 of 30 patients (56.7%).
| Treatment Modality | Immediate Angiographic Results | Imaging Follow-Up Results | Clinical Follow-Up Results | |
|---|---|---|---|---|
| Favorablea | Poorb | |||
| Stent-assisted coiling (n = 7) | Complete obliteration (n = 1, 14.3%) | Stable (n = 1, 14.3%) | (n = 2, 28.6%) | (n = 5, 71.4%) |
| Partial obliteration (n = 5, 71.4%) | Stable (n = 1, 14.3%) | |||
| Recurrence (n = 4, 57.1%) | ||||
| Intraprocedural occlusion (n = 1, 14.3%) | Stable (n = 1, 14.3%) | |||
| Stent-only therapy (n = 6) | Complete obliteration (n = 6, 100%) | Stable (n = 6, 100%) | (n = 5, 83.3%) | (n = 1, 16.7%) |
| Internal coil trapping (n = 17) | Complete obliteration (n = 16, 94.1%) | Stable (n = 13, 76.5%) | (n = 10, 58.8%) | (n = 7, 41.2%) |
| Nonec (n = 3, 17.6%) | ||||
| Partial obliteration (n = 1, 5.9%) | Recurrence (n = 1, 5.9%) | |||
aFavorable clinical outcome one year after treatment.
bPoor clinical outcome one year after treatment.
cPatients who died within several days after treatment.
Intraprocedural in-stent occlusion occurred in one patient during an attempt to perform SAC immediately after stent placement. Although the anterior-to-posterior collateral supply via a bilateral PCoA mitigated the insufficiency of cerebral blood flow into the posterior circulation, extensive cerebellar infarction occurred after the procedure. Procedure-related thromboembolic infarctions in the corresponding territory occurred in two patients after stent-assisted coil embolization; however, these infarctions were not accompanied by clinical symptoms.
Postprocedural infarction and clinical follow-up results after ICT are summarized in Table 2. A total of 16 patients with complete obliteration after ICT were enrolled in this subgroup analysis, and one patient who was treated with a modified ICT was excluded. Clinical follow-up was available in 13 patients 13 - 69 months (mean, 29.1 months) after treatment. Three patients who presented with a severe clinical condition of WFNS grade 5 expired after the procedure, probably due to aggravation of initial symptoms during hospitalization. Four patients with PICA occlusion showed corresponding PICA territorial infarctions with or without lateral medullary infarction, and two of these showed favorable clinical outcomes with mRS scores of 1 and 2, respectively. Medullary infarction occurred in three of nine dissecting aneurysms involving segments that were distal to the PICA, and two of these showed favorable clinical outcomes. Favorable clinical outcomes were achieved in 10 of 16 patients (62.5%), and three patients had mild gait disturbances and paresthesias in the lower extremities, but were still able to independently perform activities of daily living.
| Angiographic Subtype | Location | Postprocedural Infarction | Immediate Postprocedural Deatha | Clinical Follow-Up Results | |
|---|---|---|---|---|---|
| Favorableb | Poorc | ||||
| PICA-involved type (n = 4) | PICA territorial infarction (n = 4) | 1 | 2 | 1 | |
| Non-involved type (n = 12) | Distal to the PICA (n = 9) | No infarction (n = 6) | 1 | 4 | 1 |
| Medullary infarction (n = 3) | 2 | 1 | |||
| Proximal to the PICA (n = 3) | No infarction (n = 3) | 1 | 2 | 0 | |
Abbreviation: PICA, posterior inferior cerebellar artery
aPatients who died within several days after treatment.
bFavorable clinical outcome one year after treatment.
cPoor clinical outcome one year after treatment.
4.3. Predictive Factors for Favorable Long-Term Clinical Follow-Up Results
With respect to favorable long-term clinical follow-up results, the univariate analysis showed that the initial WFNS grade (P = 0.030) was a significant independent variable. The immediate angiographic result (P = 0.061) was a variable of borderline significance. In the multivariate logistic regression analysis, both initial WFNS grade (P = 0.018) and immediate angiographic results (P = 0.018) remained statistically significant predictive factors for favorable long-term clinical follow-up results. When the initial clinical condition was favorable and complete obliteration was achieved after endovascular treatment, the probability of a favorable long-term clinical outcome increased, with ORs of 16.28 (95% CI: 1.60 - 165.54) and 36.25 (95% CI: 1.87 - 702.72), respectively (Table 3).
| Variable | Category | Favorable Outcome | Poor Outcome | Punia | Plogisticb |
|---|---|---|---|---|---|
| Age, y | < 55 (n = 21) | 14 (66.7) | 7 (33.3) | 0.123 | |
| ≥ 55 (n = 9) | 3 (33.3) | 6 (66.7) | |||
| Sex | Female (n = 14) | 7 (50) | 7 (50) | 0.491 | |
| Male (n = 16) | 10 (62.5) | 6(37.5) | |||
| WFNS grade | Fair (n = 16) | 12 (75.0) | 4 (25.0) | 0.030 | 0.018 |
| Poor (n = 14) | 5 (35.7) | 9 (64.3) | |||
| Dominancy of VA | Non-dominant (n = 25) | 16 (64.0) | 9 (36.0) | 0.138 | |
| Dominant (n = 5) | 1 (20.0) | 4 (80.0) | |||
| PICA-involved type | No (n = 14) | 9 (64.3) | 5 (35.7) | 0.431 | |
| Yes (n = 16) | 8 (50.0) | 8 (50.0) | |||
| ASA-involved type | No (n = 28) | 15 (53.6) | 13 (46.4) | 0.571 | |
| Yes (n = 2) | 2 (100.0) | 0 | |||
| Treatment modality | SAC (n = 7) | 2 (28.6) | 5 (71.4) | 0.134 | |
| SOT (n = 6) | 5 (88.3) | 1 (16.7) | |||
| ICT (n = 17) | 10 (58.8) | 7 (41.2) | |||
| Immediate angiographic results | Complete (n = 24) | 16 (66.7) | 8 (33.3) | 0.061 | 0.018 |
| Partial (n = 6) | 1 (16.7) | 5 (83.3) | |||
| Posttreatment infarction | No (n = 20) | 12 (60.6) | 8 (40.0) | 0.705 | |
| Yes (n = 10) | 5 (50.0) | 5 (50.0) | |||
| Recurrence or rebleeding | No (n = 25) | 16 (64.0) | 9 (36.0) | 0.138 | |
| Yes (n = 5) | 1 (20.0) | 4 (80.0) |
Abbreviations: ASA, anterior spinal artery; ICT, internal coil trapping; SAC, stent-assisted coiling; SOT, stent-only therapy; VADA, ruptured vertebral artery dissecting aneurysmPICA, posterior inferior cerebellar artery; VA, vertebral artery; WFNS, world federation of neurosurgical societies.
aP value of univariate analysis.
bP value of multivariate analysis.
4.4. Case 1
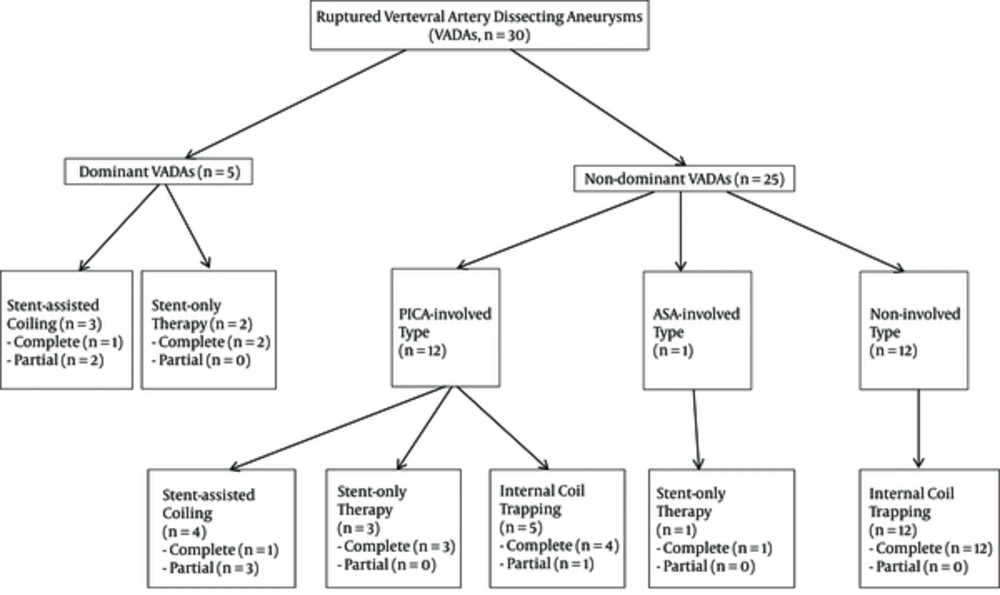
A 55-year-old male patient presented with SAH, with an initial WFNS grade of II and a right vertebral dissecting aneurysm. The left VA seemed to terminate after ramifying the PICA, and it seemed to be small in caliber. Therefore, SAC with two stents and eight detachable coils was performed to preserve the antegrade flow of both the PICA and the VA. The dissecting segment with irregular fusiform dilatation was completely occluded, and postprocedural DWIs were observed to show no infarctions in the right PICA territory. However, 13 hours after treatment, the patient had an aggravated mental state and both pupils were fixed. Follow-up brain computed tomography (CT) and DWIs revealed a recurrent hemorrhage with acute bilateral PICA territorial infarctions; the patient died two days after treatment (Figure 2).
A ruptured right vertebral dissecting aneurysm involving the dominant VA and PICA origin in a 55-year-old man. A, 3D-reformatted right vertebral angiogram reveals a dissecting PICA-involved type aneurysm; B, Left vertebral angiogram shows a hypoplastic VA with PICA termination; C, Right vertebral angiogram after SAC with a single stent showed a completely embolized aneurysmal sac with preserved antegrade flow of the PICA; D, Non-enhanced CT image, obtained 13 hours after treatment, shows an increased SAH in the posterior fossa (Abbreviations: VA, vertebral artery; PICA, posterior inferior cerebellar artery; SAH, subarachnoid hemorrhage; SAC, stent-assisted coiling).
4.5. Case 2
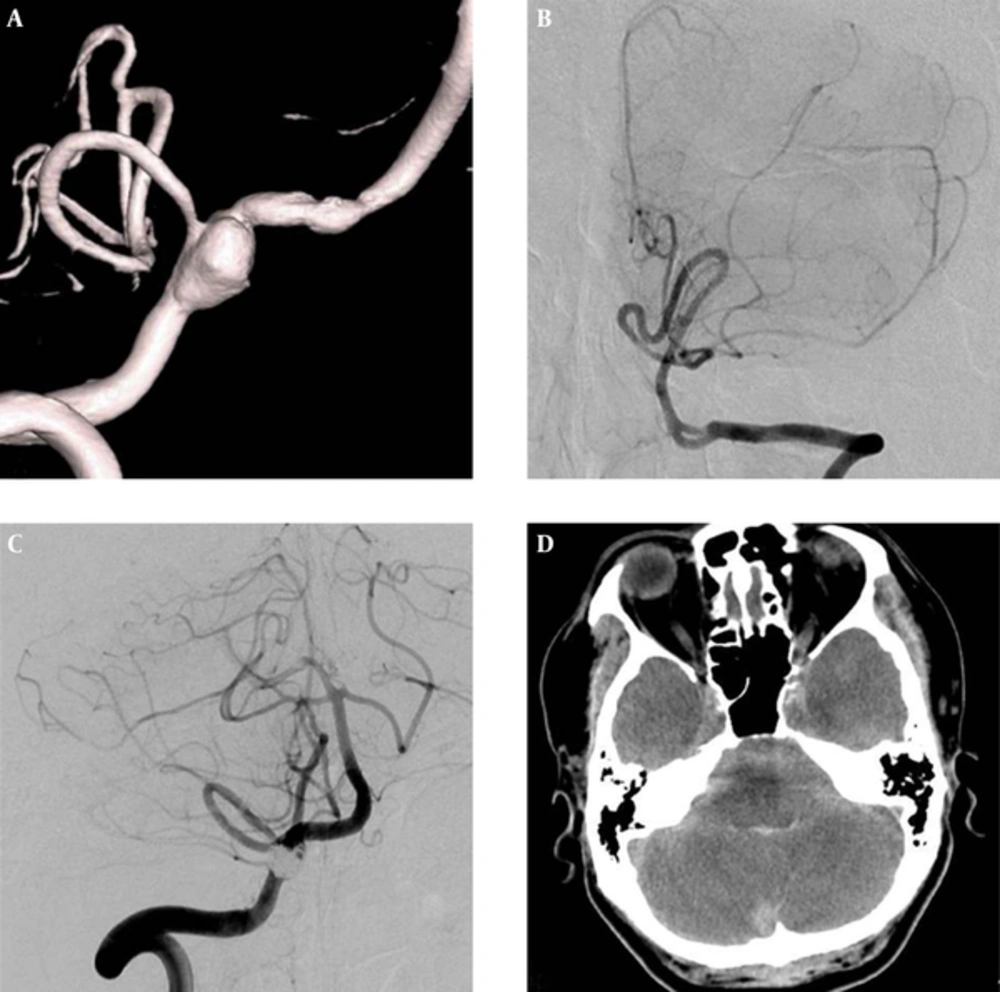
A 51-year-old female patient was admitted with SAH, with an initial WFNS grade of IV. Right vertebral angiograms showed a dissecting aneurysm involving the orifice of the ipsilateral PICA. Contralateral left vertebral angiograms revealed a left VA that was normal in caliber, but a hypoplastic PICA. Therefore, we decided to perform a modified ICT to preserve the orifice of the right PICA. Complete occlusion of the dissecting segment proximal to the PICA was achieved with eight detachable coils. Two weeks after the treatment, the patient developed an aggravated mental state, and follow-up CT revealed rebleeding. Follow-up angiograms obtained during retreatment demonstrated propagation of the dissected segment to the vertebrobasilar junction and the contralateral distal VA. Approaching the right distal VA via the left VA was not possible because of the acute angle of the vertebrobasilar junction and the small caliber of the right distal VA. Triple overlapping stents were placed in the left distal VA and the proximal basilar artery. At her 15-month follow-up appointment, the patient was severely disabled (Figure 3).
A ruptured right vertebral dissecting aneurysm involving a non-dominant VA with a PICA origin in a 51-year-old female. A, 3D-reformatted right vertebral angiogram reveals a dissecting aneurysm on a narrowed segment with a PICA origin; B, Left vertebral angiogram after modified ICT of the dissected segment proximal to the PICA shows retrograde filling of the right distal VA and PICA; C, 3D-reformatted left vertebral angiogram, obtained 14 days after the initial treatment, reveals a progressed dissecting aneurysm from the right distal VA to the contralateral distal VA and the proximal basilar artery; D, 3D-reformatted left vertebral angiogram after triple overlapping stents shows a residual dissected segment in the right distal VA (Abbreviations: VA, vertebral artery; PICA, posterior inferior cerebellar artery; ICT, internal coil trapping).
4.6. Case 3
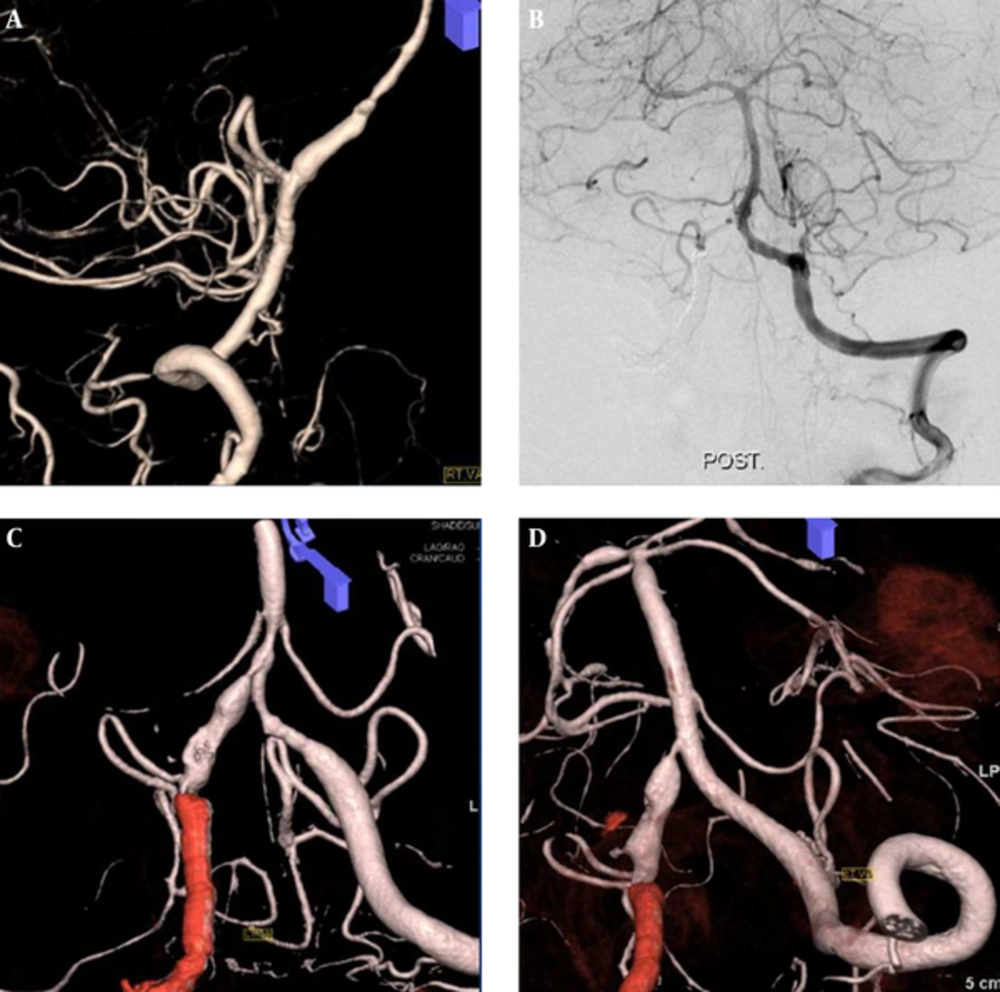
A 52-year-old female patient was referred to our hospital five days after onset of a severe headache. Brain CT images displayed a scant amount of iso- to hyperintensity in the basal cistern. Emergent vertebral angiograms showed a bi-dominant VA with a dissecting aneurysm in the right VA. On 3D-reformatted images, the dissected segment seemed to contain the orifice of the ASA. Therefore, a reconstructive technique using SOT was performed and two Enterprise stents (4 × 22 mm and 4 × 28 mm) were placed over the dissected segment and the ASA site of origin. The patient fully recovered, with no neurological symptoms, and was discharged 16 days after treatment. Follow-up DSA obtained one year after treatment showed complete occlusion of the aneurysm with a patent right VA and ASA (Figure 4).
A ruptured right vertebral dissecting aneurysm involving a non-dominant VA with an ASA origin in a 52-year-old female. A, Right vertebral angiogram reveals a dissecting aneurysm in the right VA and the ASA, arising from a dissected segment; B and C, Follow-up angiogram one year after multiple-stent placement showed complete occlusion of the aneurysm with a patent ASA (Abbreviations: VA, vertebral artery; ASA, anterior spinal artery)
5. Discussion
Prevention of recurrent hemorrhage from a ruptured VADA is the primary goal of treatment (13). The present study demonstrated that partial obliteration was significantly associated with a poor clinical outcome, and partial obliteration appears to be associated with high rates of recurrence. Specifically, remnant dissecting segments, left in place to preserve the antegrade flow of the perforating arteries, allow persistent flow through the unprotected false lumen, resulting in continuous flow to the fragile wall of the remnant pseudoaneurysm and serial enlargement or rupture (14). In a previous report, involvement of the PICA was found to be the only independent risk factor for recurrence after endovascular treatment, and this risk factor was attributed to the unprotected remnant dissecting aneurysm adjacent to the PICA (14). Furthermore, exact extension of the dissected segment in the parent artery cannot be assessed based on the existing endoluminal diagnostics. In effect, the initial dissection may extend to the orifice of the PICA or to the PICA itself. Stent-assisted coil embolization has been used frequently to preserve the patency of the parent and perforating vessels in the treatment of unruptured VADAs. However, due to the potential risk of coil compaction and remaining pathological potential in the vessel wall, this technique has been shown to be associated with incomplete obliteration and regrowth of residual aneurysms (15-17). Therefore, if a patient presents with residual aneurysmal filling on immediate postoperative angiography, short-term follow-up at 1 - 6 month intervals is recommended (18). In our study, most patients who received SAC (71.4%) had partial obliteration and progressed to recurrent hemorrhage or serial enlargement of the residual dissecting aneurysm. Given the higher recurrence rate of ruptured VADA after incomplete obliteration and the risk of a fatal clinical outcome from recurrent hemorrhage, deconstructive techniques, such as internal trapping, are the preferred treatment modalities during the acute hemorrhagic phase.
Endovascular internal trapping is an effective technique for eliminating the potential risk of rebleeding and achieving long-term durable occlusion (11, 19). Theoretically, ICT should be followed by concomitant occlusion of the parent and perforating vessels, which would result in cerebellar or brainstem infarctions. A recently published large cohort study demonstrated that occlusion of the PICA did not affect the likelihood of a favorable clinical outcome, despite being associated with an increased incidence of ischemic complications (20). In the present study, ICT was adopted for the management of PICA-involved VADAs when sufficient collateral supplies from the contralateral PICA or the ipsilateral AICA were anticipated, and posttreatment infarction was not a significant predictor for long-term favorable clinical outcomes (P = 0.705). Therefore, we speculated that deliberate sacrifice of the PICA may be less harmful than previously expected. However, there are several limitations in applying ICT to all types of ruptured VADAs. First, sacrifice of the VA is not always possible, and alternative reconstructive therapy should be considered under specific conditions, such as when the patient has a dominant vertebral dissecting aneurysm with poor collaterals. Second, clinical outcomes after occlusion of major branches are unpredictable and can vary depending on the adequacy of an individual patient’s collateral circulation. There were no data concerning the perfusion of the sacrificed PICA in the aforementioned large-scale study, and PICA revascularization procedures are required for some patients who are at high risk for ischemic complications after undergoing deconstructive therapy. Moreover, several reports have demonstrated that medullary infarction is associated with poor clinical outcomes after treatment for ruptured VADAs (13, 21). Given the fact that the segment of the VA distal to the PICA origin more frequently gives rise to perforating arteries than does the segment proximal to the PICA origin, internal trapping should be deliberately performed, even when treating a non-involved VADA with a location distal to the PICA.
Reconstructive treatment using stent-only therapy is beneficial for minimizing sacrifice of the parent and perforating vessels. Additionally, it is comparable to internal trapping for prevention of rebleeding of a ruptured VADA (10, 22-24). Consistent with many previous reports, we found promising results from stent-only therapy using multiple stents to treat ruptured VADAs with durable occlusion over a one-year follow-up period. With the advent of a neurovascular stent device, therapy with multiple overlapping stents is feasible and offers a high technical success rate. The addition of successive stent struts may effectively diminish shear stress in the aneurysm wall in proportion to the number of overlapping stents (25, 26). However, reconstructive therapy, especially stent-only therapy, does not completely eliminate the rebleeding risk. Going forward, a prospective, multi-centered study of the therapeutic effects of multiple-stent therapy and its long-term validity is required.
The retrospective nature and the small sample size of this study are the main limitations to its ability to establish an optimal endovascular treatment strategy for this challenging disease entity. Specifically, a meaningful subgroup analysis between treatment modalities was not possible due to the limited number of cases. Given the fact that the initial WFNS grade was a significant variable influencing the clinical outcome, discrepancies in preoperative statuses may induce a selection bias and have an effect on the results. However, we speculated that a logistic regression analysis with a forward stepwise method could mitigate the effect of selection bias. There is a consensus in the literature that appropriately prescribed antiplatelet medication should be mandatory for all patients who have undergone stent placement (24, 27). Therefore, an adequate postprocedural medication strategy should complement any endovascular treatment methods used.
Adequate angiographic analysis of ruptured VADAs should precede treatment, to allow for development of an optimized treatment strategy for individual patients. Given the relatively high recurrence rate of ruptured VADAs after incomplete obliteration and the association of a fatal clinical outcome from recurrent hemorrhage, prevention of rebleeding may outweigh the risk of parent and perforating artery occlusion. However, under specific conditions, reconstructive treatment using stent-only therapy could be an alternative with comparable therapeutic efficacy and long-term durability.