1. Background
Hepatocellular carcinoma (HCC) is a neoplasm that usually arises in a cirrhotic liver by a multistep carcinogenesis process. Early detection of HCC and accurate assessment of the tumor burden are crucial to successful treatment planning and long-term survival. Ultrasonography (US) and serum alpha-fetoprotein (AFP) assessment are the main screening methods for the early detection of HCC in patients with chronic liver disease and cirrhosis, even in the early stages. US has less capability for characterizing small lesions, particularly in the cirrhotic liver (1-3).
Now, HCC is often a curable tumor, so early identification of the lesions is important. Thus, the optimal strategy for HCC surveillance needs to be better characterized. Magnetic resonance imaging (MRI) plays a prominent role in the evaluation of cirrhosis and screening for early HCC. In a study, dynamic MRI with IV gadolinium injection is most sensitive for imaging HCC and best reflects the actual tumor size as a screening method; however, a conventionally performed full abdominal MRI is cumbersome as well as expensive (4, 5).
Diffusion weighted magnetic resonance imaging (DW-MRI) allows tissue characterization by probing tissue microstructural changes, quantified as the apparent diffusion coefficient (ADC). Due to the higher microstructural density in malignant lesions, water mobility and therefore, incomplete rephrasing will be less pronounced, resulting in higher signal intensity on the native DW-MRI and lower ADC compared to benign lesions. Although DWI has been claimed in some studies to be more specific and more sensitive than conventional MRI with dynamic scan in the detection of HCC nodule, there are few studies directly evaluating the potential role of DWI as a sensitive method in screening HCC (6-8).
Namazi hospital affiliated to Shiraz University of Medical Sciences in Shiraz is the center of liver transplant in Iran and it is one of the main centers in the Middle East. So, cases with liver cirrhosis are referred to this city and it helped us investigate about HCC in liver cirrhosis with a reasonable number of cases.
2. Objectives
In this study, we compared the accuracy of DWI combined with limited sequence MRI set as a potentially quick and practical MR candidate with US for screening of HCC in patients with cirrhosis.
3. Patients and Methods
3.1. Patient Enrollment
Inclusion criteria were cirrhotic patients who were referred to the gastroenterologist for follow-up during the period of the study. Ninety-six of these patients were selected. Thirty of them who had concomitant hepatocellular carcinoma (HCC) proved by pathology were chosen. Sample volume was determined by an expert biostatistician, considering previous associated studies with an alpha value equal to 5% and power equal to 85%. Radiologists were blinded for definite diagnosis of the patients. Selection was by simple sampling among the patients referred by gastroenterologists to Faghihi hospital affiliated to Shiraz University of Medical Sciences from Dec. 2011 to Jan. 2013. Non-cooperative cases, severe ascites, and contraindications for MRI were excluded from the study.
3.2. Imaging Techniques
MRI, DWI, and US of the liver were performed for the patients. All MR examinations were performed using the same fast limited protocol, with a 1.5-T clinical MR unit (Magnetom, Avanto, Siemens Healthcare, Erlangen, Germany) with an 8-channel body phase-array coil. The liver was imaged in the axial plane in all sequences. Baseline MR images included axial half-Fourier acquisition single-shot turbo spin-echo (HASTE) images (TR/TE; 1800/72; 150° refocusing flip angle; 256 × 129 matrix; 6 mm slice thickness) and a breath-hold T1-weighted fast low angle shot (FLASH) sequence (a double echo chemical shift gradient echo sequence) (TR/first echo TE, second echo TE, 70/2.5 [opposed phase (OP)], 5 [in phase (IP)]; 70° flip angle; matrix of 256 × 137; 6 mm slice thickness; signal average one; and two acquisitions). After baseline MRI, a respiratory triggered single-shot fat-suppressed echo-planar DWI sequence in the axial plane with prospective acquisition correction was acquired using TR/TE, 2100/85 ms; 90° flip angle; 6 mm slice thickness; and matrix, 192 × 115. The gradient factors (b values) were 50, 400, and 800 s/mm2. Depending on the respiratory efficiency of each patient, the acquisition time for this sequence ranged from 2 to 4 min. To improve the image quality, integrated parallel imaging technique (iPAT) by means of generalized auto-calibrating partially parallel acquisitions (GRAPPA) with an acceleration factor of 2 was applied (9). The total slices of DW-MR images were different in the base of liver length. The slice gap and field of view were occasionally changed according to the size of the liver to ensure coverage of the whole liver.
Quantitative ADC maps were created on a voxel-by-voxel basis using the algorithms implemented within the Siemens Magnetom scanner software and using the b values of 50, 400, and 800 s/mm2. On DWI, a lesion was considered malignant when it was moderately hyperintense at b 50 s/mm2, remained hyperintense at b 400 s/mm2 and b 800 s/mm2, and showed an ADC value that was equal to or lower than that of adjacent liver parenchyma (10). The MRI, DWI, and a combination of them were interpreted independently by two abdominal radiologists with 7 and 8 years of experience. Kappa correlation coefficient was compared between the two reports.
US of the liver was performed for each patient by Logic 7, GE, USA, ultrasound machine, with a 3.5 MHz curve transducer and 7.5 MHz linear probe for surface evaluation. US was done by a radiologist with ten years of experience in abdominal US. He determined whether the lesion suspected of HCC existed or not. Histopathological results of the lesion biopsies were considered as reference standard.
3.3. Statistical Analysis
In order to assess interobserver agreement for the evaluation of DWI and conventional MRI, we calculated the kappa statistics for the two mentioned radiologists (11). Kappa values between 0.41 and 0.60 indicate moderate agreement,between 0.61 and 0.80 good agreement, and values greater than 0.81 indicate excellent agreement. Sensitivity, specificity, and accuracy of DWI alone, limited sequences of MRI alone, combination of them as well as US for detection of HCC in these patients were calculated. Then comparison between all modalities was performed using descriptive statistics and the McNemar test. A P value less than 0.05 was considered as statistically significant. All statistical analyses were conducted using SPSS ver. 16.0 (SPSS Inc., Chicago, IL, USA).
4. Results
Of the 96 patients with cirrhosis, US showed 21 cases with nodule/mass suggestive of HCC. It determined 12 cases with indeterminate report as “possibility of HCC cannot be ruled out” due to poor acoustic window (obesity or gas distended bowel loops), and severe coarse echogenicity and nodularity of the liver parenchyma. In 63 cases, no evidence for HCC was noted. Finally US revealed six false negative and three false positive cases. Therefore, US had a sensitivity of 60%, specificity of 86.36%, and accuracy of 78.12%.
MRI and DWI were reported by two observers. With regard to inter-observer agreement, the kappa value of the limited fast MRI alone was 0.745, indicating a good inter-observer agreement. The value of DWI alone was 0.713, which indicates a good inter-observer agreement. Also kappa value for the combination of MRI and DWI was 0.85, indicating an excellent inter-observer agreement. In MRI results by observer 1, there were five false negative and 10 false positive with a sensitivity of 83.33%, specificity of 84.84%, and accuracy of 84.37%. For observer 2, there were eight false negative, and eight false positive with a sensitivity of 73.33%, specificity of 87.77%, and accuracy of 83.33%. From MR sequences, chemical shift T1 weighted images (OP and IP) showed the highest sensitivity for the detection of lesions but not necessarily HCC. Then correlation with HASTE images could suggest the diagnosis.
For observer 1, DWI showed eight false negative cases. However, there was no false positive case with a sensitivity of 73.33%, specificity of 100%, and accuracy of 91.66%. For observer 2, DWI revealed seven false negative without false positive with a sensitivity of 76.66%, specificity of 100%, and accuracy of 92.75%.
Combination of MRI and DWI showed five false negative cases without false positive for both observers with a sensitivity of 83.33%, specificity of 100%, and accuracy of 94.79%.
Comparison between sensitivity, specificity, and accuracy of the US, MRI, DWI, and combination of MRI and DWI revealed the best results for combination of limited sequences MRI set and DWI (Table 1).
| US | MRI, Observer1 | MRI, Observer2 | DWI, Observer1 | DWI, Observer2 | MRI + DWI, Observer1 | MRI + DWI, Observer2 | |
|---|---|---|---|---|---|---|---|
| Sensitivity | 60 | 83.33 | 73.33 | 73.33 | 76.66 | 83.33 | 83.33 |
| Specificity | 86.36 | 84.84 | 87.77 | 100 | 100 | 100 | 100 |
| Accuracy | 78.12 | 84.37 | 83.33 | 91.66 | 92.75 | 94.79 | 94.79 |
| Positive Predictive Value | 85.71 | 71.42 | 73.33 | 100 | 100 | 100 | 100 |
| Negative Predictive Value | 90.47 | 91.80 | 87.87 | 89.19 | 90.41 | 92.96 | 92.96 |
aAll numbers are presented as percent.
5. Discussion
Cirrhosis is the main risk factor for HCC, and screening of HCC in patients with cirrhosis is momentous. Detection of an early stage HCC can help save the patients by using new treatments. We compared US, MRI, and DWI in the screening of HCC in cirrhotic patients. There was a higher accuracy in detecting HCC when limited sequences MRI and DWI were used together. This method lasted for about 10 minutes. So it could be an alternative screening method for HCC in cirrhotic patients.
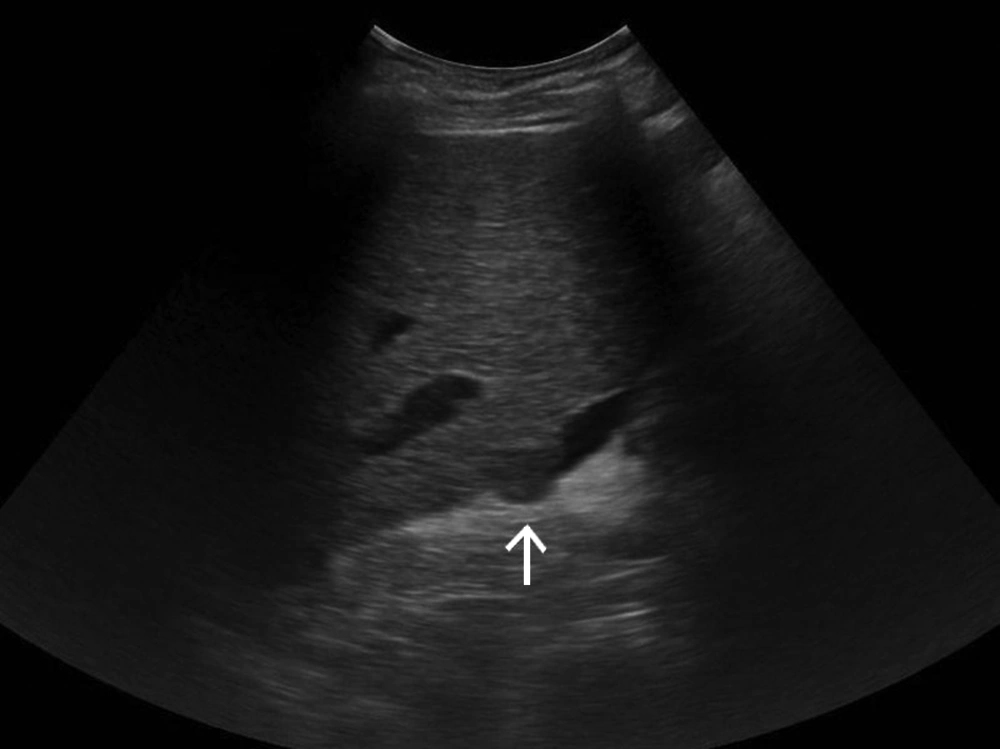
Increase in the prevalence of liver cirrhosis followed by a high prevalence of HCC in this background has led to a prominent role for screening of HCC. US and AFP have the main role for this purpose. In addition to poor acoustic window in some patients due to obesity or gas distended hollow viscus, coarse echogenicity and nodularity of most cirrhotic livers limit US detection of small neoplastic nodules. Also US performed by technicians or other specialists without sufficient skill is another problem of this operator-dependent modality. Based on the above-mentioned facts, US has limited reproducibility; therefore, we are introducing an alternative method with better diagnostic accuracy (Figure 1).
In contrast to our study, Irshad et al. said that despite technological improvements with high-speed/high-resolution CT and MR capabilities, because of the cost-effectiveness of US, it still keeps a preferred place worldwide for routine screening of HCC in patients with known liver disease. Increased resolution of the newer US equipment and the use of new techniques have enabled the sonographers to find smaller nodules and subtle changes in the liver texture with increased accuracy (12).
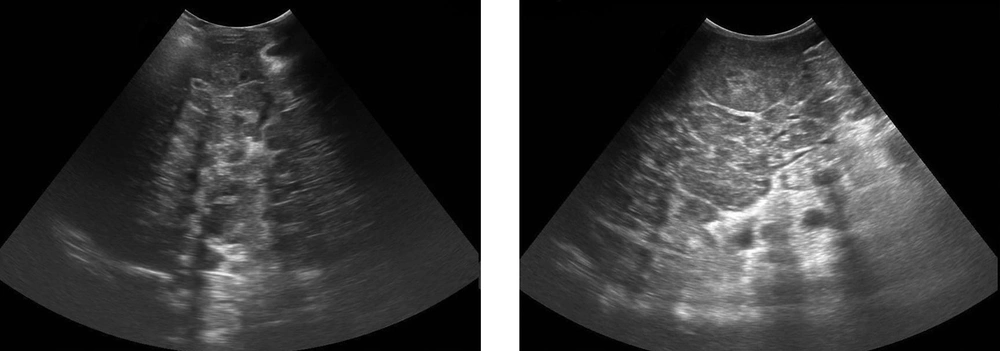
However, another study reported the limitations of US such as previously mentioned operator dependency, inability to detect lesions smaller than 1 cm in size, and low specificity. The presence of diffuse liver disease (as in the present study) also lowers the sensitivity of US for detection of focal lesions. Similarly, pseudolesions, such as focal fatty infiltrations or focal fatty sparing, are sometimes difficult to differentiate from other pathologic liver lesions (13) (Figure 2).
In most studies, dynamic-enhanced MRI was the choice for detection of HCC; however, some studies suggested DWI as a complementary method.
In two studies conducted by Xu et al. and Park et al. using combined gadolinium-enhanced MRI and DWI made it possible to reliably diagnose small HCC including hypovascular HCCs in cirrhotic patients. In a meta-analysis study of nine articles about DWI in the diagnosis of HCC, the comparison of DWI performance with that of conventional contrast enhanced MRI (CE-MRI) suggested no major differences between these two methods. DWI combined with CE-MRI had a higher sensitivity than DWI alone (14-16).
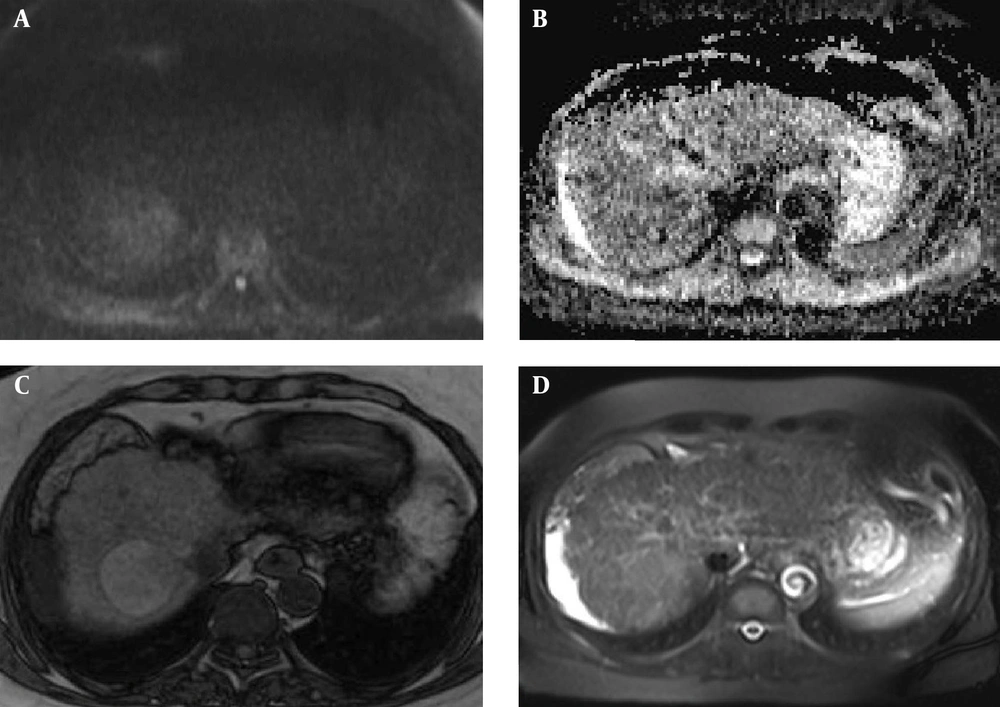
In our study, CE-MRI was not performed because we wanted to provide a non-invasive and fast method used for screening of HCC in the cirrhotic liver. DWI was a good choice because of its good accuracy in previous mentioned studies and its performance without contrast media. Also, we selected two fast sequences (IP/OP T1W and HASTE) for MRI, so their better spatial resolution led to better detection of the nodules. This study showed increasing diagnostic accuracy of DWI with a combination of these sequences (Figure 3).
As a result, due to significant improvements in the treatment of early stage HCC compared to even the previous decade, we suggest a fast, non-invasive and more accurate method (HASTE, OP/IP T1W sequences of MRI combined with DWI) rather than US for screening of HCC in liver cirrhosis. This method is also less time consuming and less expensive than the routine MRI with usual liver protocol. However the suggested method’s cost-effectiveness compared to US should be investigated in other studies.