Abstract
Background:
Patients with hematologic diseases are frequently accompanied by coagulopathy; even a small amount of hemoptysis may worsen clinical outcomes compared to those with the similar severity of injury without coagulopathy. However, a few studies have included various hematologic conditions, and none of them were large series.Objectives:
To evaluate the feasibility and short-term efficacy of bronchial artery embolization (BAE) in the management of hemoptysis in patients with hematologic diseases.Patients and Methods:
This was a retrospective study of 25 patients with hematologic diseases who visited our interventional unit for the management of hemoptysis between 2009 and 2012. The clinical, laboratory, and radiographic data were retrospectively analyzed and we correlated clinical response with the amount of hemoptysis, coagulation condition, radiographic pattern, and bronchial artery hypertrophy.Results:
The amount of hemoptysis was trivial in 13 patients (52 %), moderate in ten patients (40%), and massive in two patients (8%). Thirteen patients (52%) had coagulopathy (platelet count < 5 × 104 µL and/or international normalized ratio (INR) > 1.5). Seventeen patients (68%) showed focal pulmonary hemorrhagic patterns and eight patients (32%) showed diffuse pulmonary hemorrhagic patterns. BAE provided complete clinical response in 21 out of 25 patients (84%). Complete clinical response was not correlated with the amount of hemoptysis, coagulation condition, radiographic pattern, or bronchial artery hypertrophy (P > 0.05). There was no significant difference in short-term survival between patients with coagulopathy and those without coagulopathy (P = 0.425), and between patients with focal hemorrhagic pattern and those with diffuse hemorrhagic pattern (P = 0.728).Conclusion:
BAE in hematology patients was relatively efficient in controlling hemoptysis. The amount of hemoptysis, coagulation condition, radiographic pattern, or bronchial artery hypertrophy was not a significant factor affecting the outcome.Keywords
1. Background
Although hemoptysis can be induced by a wide variety of diseases, massive hemoptysis is most frequently caused by infections such as pulmonary tuberculosis, bronchiectasis, aspergillosis, or lung abscess. In addition, patients with cystic fibrosis or lung cancer are associated with massive hemoptysis. Most of the previous studies about bronchial arterial embolization (BAE) have focused on the treatment of life-threatening massive hemoptysis associated with benign pulmonary diseases or neoplastic lesions (1-3). However, since patients with hematologic diseases are frequently accompanied by coagulopathy that is known as a negative factor in hemorrhage control, even a small amount of hemoptysis may worsen clinical outcomes more than those with the similar severity of injury without coagulopathy (4-6).
Although several studies have reported on BAE for hemoptysis in hematologic diseases, most studies have been published as case reports on immunocompromised conditions, such as pulmonary fungal infection or tuberculosis (7, 8). A few studies have included various hematologic conditions, and none of them were large series.
2. Objectives
The focus of this study was to verify the feasibility and short-term efficacy of BAE in the management of hemoptysis in patients with hematologic diseases. More specifically in terms of the clinical response, we correlated it with the amount of hemoptysis, coagulation condition, radiographic pattern and bronchial artery hypertrophy.
3. Patients and Methods
This retrospective study was approved by our institutional review board (CMC clinical research coordination center, the catholic University of Korea). The written informed consent was specially waived by the approving institutional review board and patient information was anonymized and de-identified prior to analysis. We reviewed 28 patients with hematologic diseases (17 men, 11 women; mean age, 54 years (range, 27 - 68 years)) who were referred to our interventional unit for the management of hemoptysis between 2009 and 2012. All patients had the first episode of hemoptysis. Fifteen patients had acute myelogenous leukemia (AML, 54%), four patients had myelodysplastic syndrome (15%), three patients had acute lymphocytic leukemia (ALL, 11%), two patients had chronic myelogenous leukemia (CML, 7%), one patient had chronic lymphocytic leukemia (CLL, 3%), two patients had multiple myeloma (7%), and one patient had idiopathic thrombocytopenic purpura (ITP, 3%). All patients underwent chest radiographs and computed tomography (CT) to determine the etiology and extent of pulmonary disease, and the origin of bleeding. The presence of underlying pulmonary diseases was determined on the basis of clinical history, laboratory test results, and radiological findings.
In this study, abnormal values for platelet count (< 5 × 104 µL and/or international normalized ratio (INR) > 1.5) were defined as coagulopathy because these values require correction. Free-frozen plasma or platelet transfusion was given to patients with coagulopathy during the procedure. The decision to treat by embolization was made by the hematology physicians in consultation with the interventional radiologists.
The amount of hemoptysis was classified as trivial (blood-tinged sputum), moderate (frank blood < 300 mL per 24 hours), or massive (frank blood > 300 mL per 24 hours). According to guidelines for percutaneous transcatheter embolization established by the society of interventional radiologists (SIR) standards of practice committee (9), clinical response was determined by using results within 1 month following the embolization procedure and was classified as follows: complete clinical response (hemoptysis resolved without recurrence) or no change (persistent hemoptysis associated with a positive effect on the clinical course). In each patient, the end of follow-up was defined as the date of death, date of last contact, or 6 months after BAE.
3.1. Radiologic Findings
Chest computed tomography (CT) scans were taken within 1 week before the procedure in all patients. They revealed diffuse pulmonary hemorrhagic patterns in 11 patients (39%) and focal pulmonary hemorrhagic patterns in 17 patients (61%). Diffuse pulmonary hemorrhagic pattern was defined as diffuse consolidation or ground-glass opacity without underlying parenchymal abnormalities. Focal pulmonary hemorrhagic patterns included focal pneumonia (n = 7), invasive aspergillosis (n = 7, aspergillic antigen positive), active tuberculosis (n = 1), non-tuberculous mycobacterial infection (n = 1), and focal mass in multiple myeloma (n = 1).
3.2. Bronchial Arterial Embolization Procedures
BAE was carried out in a dedicated vascular interventional suite equipped with digital subtraction technology by three experienced interventional radiologists. In all cases, a preliminary descending thoracic aortogram was obtained initially to evaluate hypertrophied bronchial and non-bronchial systemic arteries and identify the bleeding point. In addition, the routine selective arteriography of the internal thoracic and subclavian arteries on the affected side was performed to detect collaterals feeding the bleeding sites. A 5-French angiographic catheter (Bronchial, Seong-Won Medical, Seoul, Korea) was then selectively introduced into the hypertrophied artery, and selective angiography was conducted. The angiography findings corresponding to hemoptysis were determined as follows: hypertrophic and tortuous bronchial arteries, hypervascular parenchymal staining, or systemic-pulmonary shunting. For embolization of pathologic arteries, a microcatheter (1.9 Fr, Asahi, Japan and 2.0 Fr, Terumo, Japan) was accessed superselectively as close as possible to distal embolization. Selective catheterization and embolization of the bronchial arteries was attempted even in cases with apparently normal thoracic aortograms because bleeding may occur from normal-diameter arteries (10).
Polyvinyl alcohol (PVA) particles of 350- to 500-µm diameter (Contour, Boston Scientific, Watertown, USA) were used as embolic materials. PVA particles were slowly injected under fluoroscopic control until blood flow reached complete stasis. Selective angiography and descending thoracic aortography were performed after BAE to confirm interruption of arterial flow. The puncture site was sealed with a vascular closure device (Angio-Seal; St. Jude Medical, St. Paul, MN, USA).
3.3. Statistical Analysis
SPSS for Windows version 19.0 (SPSS, Inc, Chicago, Illinois), was used for statistical analysis. All data were tabulated as mean and standard deviation in case of quantitative variables as absolute numbers and percentages in the case of qualitative variables. Fisher’s exact test was used to calculate the association between variable predictive factors (amount of hemoptysis, bronchial arterial hypertrophy, coagulopathy and CT findings). Survival curves between the groups were constructed according to the Kaplan-Meier method. Survival curves were compared using the log-rank test.
4. Results
4.1. Clinical Presentation and Response
The embolization procedure was technically successful in 25 patients (89%) and unsuccessful in three patients (11%). Three patients were excluded because they underwent only diagnostic angiography without embolization due to inability to correctly place the 5-Fr catheter tip at the origin of the bronchial arteries. After the first procedure, the possible causes of hemoptysis were determined on the basis of the review of radiographic and laboratory data.
Table 1 shows underlying hematologic disease, patient’s clinical and radiologic conditions and outcomes. After embolization, bleeding stopped within the first 24 hours in 21 patients (84%). The overall clinical success rate was 80% (20 out of 25 patients showed complete clinical response). Recurrent hemoptysis occurred in one patient (4%). This patient who had multiple myeloma and underlying bronchiectasis in the left lower lobe was medically managed and eventually resolved without reintervention. The 30-day mortality rate was 24% (6 out of 25 patients) and the 6-month survival rate was 68% (17 out of 25 patients). Of the six patients who died within 30 days after BAE, the main causes of death were (1) uncontrolled pulmonary hemorrhage after successful embolization in four patients, (2) intracranial hemorrhage due to uncontrolled coagulopathy in one patient (died 8 days after BAE), and (3) hepatic failure (died 1 month after BAE). There was no procedure-related major complication, according to the SIR definitions (9). The puncture site was successfully sealed without complications using a vascular closer device.
Summary of Data in the Patients and Clinical Results of Bronchial Artery Embolization (BAE)a
| Patient No. | Hematologic Disease | Amount of Hemoptysis | Arterial Hypertrophy | Radiologic Pattern | Coagulopathy | Response | Short-Term (6 Months) Outcome |
|---|---|---|---|---|---|---|---|
| 1 | ITP | Trivial | No | FPH | + | Complete | Died of intracranial hemorrhage, 8 days |
| 2 | AML | Trivial | Yes | DPH | + | Complete | Alive |
| 3 | AML | Trivial | Yes | DPH | + | No change | Died of persistent hemorrhage, the day after embolization |
| 4 | MDS | Moderate | Yes | DPH | + | Complete | Alive |
| 5 | AML | Trivial | No | DPH | + | Complete | Alive |
| 6 | AML | Moderate | No | DPH | + | Complete | Alive |
| 7 | MDS | Massive | No | DPH | + | Complete | Alive |
| 8 | AML | Trivial | No | FPH | + | Complete | Alive |
| 9 | AML | Moderate | Yes | FPH | + | Complete | Alive |
| 10 | MM | Trivial | Yes | FPH | + | Complete | Alive |
| 11 | MDS | Massive | Yes | DPH | 6.1 | No change | Died of persistent hemorrhage, 20 days after embolization |
| 12 | MDS | Moderate | No | FPH | + | Complete | Alive |
| 13 | AML | Moderate | Yes | FPH | 17.2 | Complete | Alive |
| 14 | AML | Trivial | Yes | FPH | 7.9 | Complete | Died of sepsis, 6 months after embolization |
| 15 | AML | Trivial | No | FPH | + | Complete | Alive |
| 16 | ALL | Trivial | Yes | FPH | 18.6 | Complete | Died of hepatic failure, 1 month after embolization |
| 17 | ALL | Moderate | No | DPH | 11.8 | Complete | Died of pneumonia, 6 months after embolization |
| 18 | MM | Moderate | No | FPH | 28.1 | Complete | Alive |
| 19 | AML | Trivial | Yes | FPH | 6.8 | No change | Died of persistent hemorrhage, the day after embolization |
| 20 | CLL | Moderate | Yes | FPH | 19.0 | Complete | Alive |
| 21 | AML | Moderate | Yes | FPH | + | No change | Died of persistent hemorrhage, 5 days after embolization |
| 22 | ALL | Trivial | Yes | FPH | 20.6 | Complete | Alive |
| 23 | CML | Trivial | Yes | FPH | 20.6 | Complete | Alive |
| 24 | CML | Moderate | Yes | FPH | 9.7 | Complete | Alive |
| 25 | AML | Trivial | Yes | FPH | 43.6 | Complete | Alive |
4.2. Coagulation Condition
On admission of 25 patients, laboratory tests revealed a mean platelet count of 9.6 ± 9.9×104/µL (range, 5.1 - 43.6×104/ µL) and mean INR value of 1.26 ± 0.23 (range, 0.96 - 2). Among these patients, 13 (52%) had coagulopathy (platelet count < 5 × 104 µL and/or INR > 1.5); of these 13 patients, 11 (85%) showed complete clinical response, and two (15%) showed no response after BAE. Of the 12 patients without coagulopathy, ten (83%) showed complete clinical response, and two (17%) showed no response (Table 2). There was no significant difference in the clinical response between the two groups (P > 0.05).
Coagulation Condition and Responsea
| Coagulation Condition and Response | No. of Patients (N = 25) |
|---|---|
| Coagulopathy condition | 13 (52) |
| Complete clinical response | 11 (85) |
| No change | 2 (15) |
| Noncoagulopathy condition | 12 (48) |
| Complete clinical response | 10 (83) |
| No change | 2 (17) |
The 6-month survival rates in patients with coagulopathy and without coagulopathy were 77% and 58%, respectively. A marginally higher survival rate was noted in patients with coagulopathy, but this did not reach statistically significance (P = 0.425, long rank = 0.635).
4.3. Radiographic Findings
The angiographic findings corresponding to hemoptysis included hypertrophic and tortuous bronchial arteries (64%, 16 out of the 25 patients), hypervascular parenchyma staining (56%, 14 out of the 25 patients), and systemic-pulmonary shunting (40%, 10 out of the 25 patients). The arteries that were hypertrophied on diagnostic angiography included bronchial arteries (n = 16), intercostal arteries (n = 7), and internal mammary arteries (n = 5).
Bronchial artery hypertrophy was defined as a bronchial artery larger than adjacent normal intercostal arteries. Of 25 patients, 16 (64%) showed bronchial artery hypertrophy and nine (36%) showed bronchial artery of normal diameter on preliminary descending thoracic aortograms. All of 25 patients underwent successful embolization. Of the 16 patients with bronchial artery hypertrophy, 12 (75%) had complete clinical response. All of the nine patients with non-hypertrophied bronchial arteries showed complete clinical response. The presence of bronchial artery hypertrophy had no statistically significant effect on the 30-day clinical outcome (P > 0.05)
Of the 17 patients with focal pulmonary hemorrhagic patterns on chest CT, 12 (71%) had bronchial artery hypertrophy. Of the eight patients with diffuse pulmonary hemorrhagic patterns, four (50%) had bronchial artery hypertrophy. The frequency of bronchial artery hypertrophy was significantly higher in patients with focal pulmonary hemorrhagic patterns than in those with diffuse pulmonary hemorrhagic patterns (P < 0.05).
Complete clinical response was observed in 15 patients (88%) with focal pulmonary hemorrhagic patterns and six patients (75%) with diffuse pulmonary hemorrhagic patterns (Table 3). There was a slightly higher rate of complete clinical response in patients with focal hemorrhagic patterns; however, this tendency was not statistically significant (P > 0.05). There was no statistically significant difference in the survival rate between focal and diffuse pulmonary hemorrhagic patterns (P = 0.728, long rank = 0.121) (Figure 1).
Radiologic Findings and Responsea
| Radiologic Findings and Response | No. of Patients (N = 25) |
|---|---|
| Focal pulmonary hemorrhagic pattern | 17 (68) |
| Complete clinical response | 15 (88) |
| No change | 2 (12) |
| Diffuse pulmonary hemorrhagic pattern | 8 (32) |
| Complete clinical response | 6 (75) |
| No change | 2 (25) |
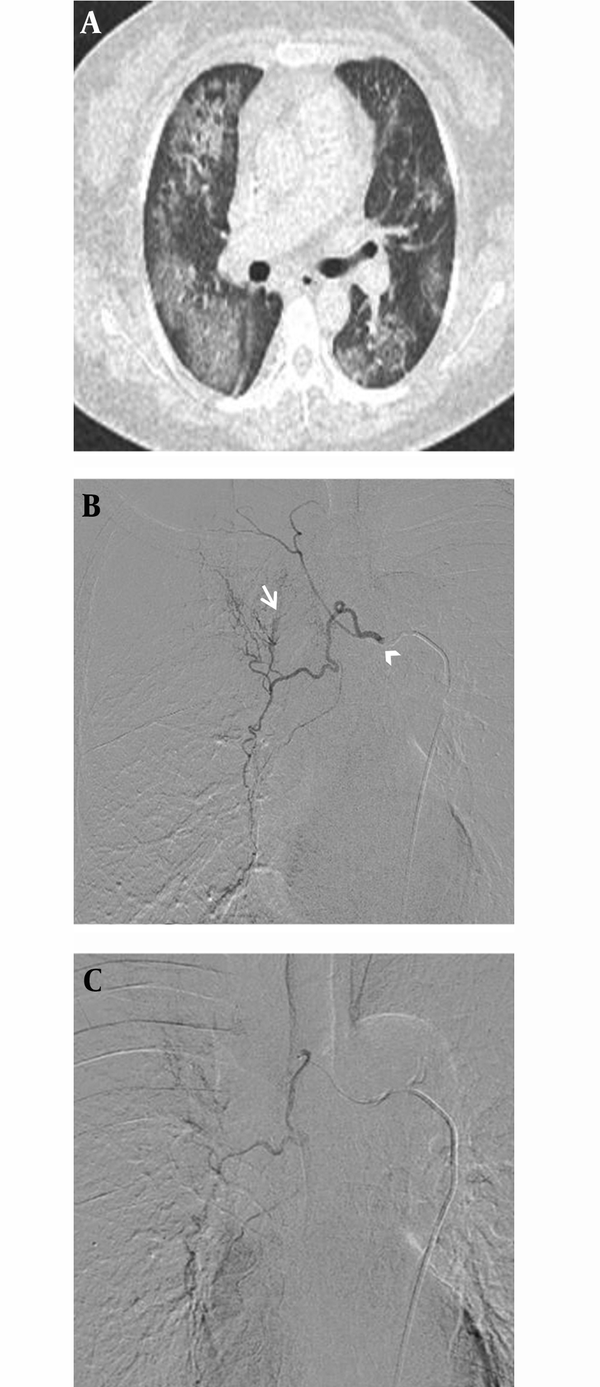
A, Diffuse pulmonary hemorrhagic pattern in a 47-year-old female with acute myelogenous leukemia. This patient was referred for management of moderate hemoptysis. Chest CT shows bilateral patchy areas of ground glass opacity without underlying pulmonary abnormality. B, Selective digital subtraction angiography image demonstrates hypertrophy in the right intercostobronchial trunk (arrow head) with systemic-pulmonary shunting (arrow). C, Superselective catheterization of the right bronchial artery with a 1.9 Fr microcatheter. Immediate cessation of hemoptysis was obtained after embolization of this artery.
Correlation Between Clinical Response and Various Predictive Factors
| Outcome | Bronchial Artery Embolization Outcomes in 25 Hematologic Patients | ||
|---|---|---|---|
| 30-Day Clinical Success | |||
| Complete Response | No Response | P Value | |
| Amount of hemoptysis | 0.546 | ||
| Trivial | 11 | 2 | |
| Moderate - Massive | 10 | 2 | |
| Bronchial artery hypertrophy | 0.332 | ||
| Yes | 12 | 4 | |
| No | 9 | 0 | |
| Coagulation condition | 0.245 | ||
| With | 11 | 2 | |
| Without | 10 | 2 | |
| Radiologic pattern | 0.752 | ||
| Focal | 15 | 2 | |
| Diffuse | 6 | 2 | |
5. Discussion
Most of the published reports on BAE have focused on patients with inflammatory causes of hemoptysis or tumor-related bleeding (1-3). However, a few reports have included various hematologic condition and none of those were large series. This study was designed to evaluate the short-term efficacy of BAE in the management of hemoptysis in patients with hematologic diseases, who had complex underlying medical issues that may influence the overall survival and limit the response to invasive procedures. In our study, although the clinical success rate was as high as 80%, eight patients (32%) died within 6 months and the 30-day mortality rate was 24%. Nevertheless, this study demonstrates that BAE may be an effective palliative tool in patients with hematologic diseases because most of the patients had no recurrent hemoptysis until the time of death after the successful BAE procedure. The causes of death included bleeding in another site, hepatic failure, and pneumonia or sepsis, not persistent hemoptysis. In four patients, hemoptysis could not be controlled with BAE and all of the four patients died within 20 days due to persistent hemoptysis, along with decreased lung function. This may reflect the clinical implication that any additional stress including uncontrolled hemoptysis can herald a terminal event in hematologic patients. Thus, we suppose that patients with hematologic diseases should be aggressively managed even when they have mild hemoptysis.
Selective catheterization of the bronchial arteries should be attempted even in cases with an apparently normal aortogram because bleeding may occur from normal-diameter vessels (10). Moreover, a moderate degree of hemoptysis and even a mild degree of hemoptysis are considered indications for transcatheter embolization (11). Urgent aggressive treatment is necessary in patients with hematologic diseases because they have complex underlying diseases and because any additional stress can influence clinical course. In our study, complete clinical response was observed in all nine patients with non-hypertrophied bronchial arteries who underwent BAE. We believe that prophylactic embolization should be undertaken in patients with non-hypertrophied bronchial arteries because it can improve clinical course and survival, although prognosis also depends on the hematologic conditions of patients in addition to success in hemostasis.
Aina et al. (12) reported that patients with coagulopathies after transcatheter arterial embolization experience recurrent hemorrhage more frequently than those without. However, the recurrence rate of hemoptysis in our series was 4% (only one patient). In addition, clinical success rate of BAE was high regardless of the presence of coagulopathy. These results imply that BAE may be efficient in ceasing arterial bleeding and can be the first treatment option for hemoptysis in hematology patients, even in the presence of coagulopathy.
Diffuse pulmonary hemorrhage has been reported in immunocompromised patients, such as those with hematologic malignancy (13-17). In general, diffuse alveolar hemorrhage is a hemorrhagic syndrome, but it is not associated with hemoptysis because the alveolar epithelium is not just leaking fluid but is perforated sufficiently to leak red cells (18). Thus, aggressive correction of thrombocytopenia and coagulation disturbances may be the first and sufficient treatment option (18). Massive pulmonary hemorrhage that can induce hemoptysis is an uncommon complication of leukemia and is generally attributed to thrombocytopenia. However, a platelet deficiency cannot be the only explanation since most patients with thrombocytopenia do not experience this complication (19). Smith et al. (19) showed that the combination of severe thrombocytopenia and diffuse alveolar damage is required for the development of massive intra-alveolar hemorrhage in patients with leukemia. Many chemotherapeutic agents, sepsis, or transplantation can cause diffuse alveolar damage. Our study includes eight patients who had hemoptysis along with diffuse pulmonary hemorrhagic patterns on CT and blood in the lavage specimens. Despite the uncorrected coagulopathy,75% of the eight patients with diffuse pulmonary hemorrhagic pattern showed complete clinical response. These results imply that BAE may be an effective treatment option for patients with diffuse pulmonary hemorrhagic patterns. Therefore, BAE may be helpful in correcting the bleeding defect and preventing further lung damage.
In general, emergent medical therapy is the first treatment option in hematologic patients with coagulopathy and diffuse alveolar hemorrhage (18). However, in our results, there was no statistically significant difference in clinical success rate and survival rate between patients with coagulopathy or diffuse alveolar hemorrhagic pattern and other hematologic patients. (Table 4). Regardless of the presence of coagulopathy or diffuse alveolar hemorrhage pattern, a high clinical success rate and low recurrence rate were achieved in patients who were treated with BAE.
This study had limitations. First, this study was of retrospective nature and had a small sample size without randomization. Second, this study was limited to patients who underwent BAE. There were no comparisons between patients undergoing BAE and those who were treated with conservative treatment alone. Despite the effectiveness of BAE, its superiority over conservative therapy in stopping hemoptysis and reducing morbidity remains unproven. Only prospective randomized trials can resolve this issue. However, the results of this study demonstrated that the frequency of complete clinical response became higher after BAE regardless of the correction of coagulopathy. In addition, BAE is a minimally invasive and relatively safe procedure. Therefore, we suppose that BAE may be the first treatment option in hematology patients with hemoptysis.
Transcatheter arterial embolization for hemoptysis in patients with hematologic disease was efficient for ceasing hemoptysis. Presence of coagulopathy or differentiation of radiographic patterns were not significant factors affecting clinical outcomes. Our study demonstrates an immediate decrease or resolution in bleeding in 80% of the patients and a low recurrence rate of 4% (only one patient). These results suggest that BAE may be an excellent treatment option for hemoptysis in patients with hematologic diseases.
References
-
1.
Fellows KE, Khaw KT, Schuster S, Shwachman H. Bronchial artery embolization in cystic fibrosis; technique and long-term results. J Pediatr. 1979;95:959-63.
-
2.
Knott-Craig CJ, Oostuizen JG, Rossouw G, Joubert JR, Barnard PM. Management and prognosis of massive hemoptysis. Recent experience with 120 patients. J Thorac Cardiovasc Surg. 1993;105(3):394-7. [PubMed ID: 8445918].
-
3.
Rabkin JE, Astafjev VI, Gothman LN, Grigorjev YG. Transcatheter embolization in the management of pulmonary hemorrhage. Radiology. 1987;163(2):361-5. [PubMed ID: 3562815]. https://doi.org/10.1148/radiology.163.2.3562815.
-
4.
Spahn DR, Cerny V, Coats TJ, Duranteau J, Fernandez-Mondejar E, Gordini G, et al. Management of bleeding following major trauma: a European guideline. Crit Care. 2007;11(1):R17. [PubMed ID: 17298665]. https://doi.org/10.1186/cc5686.
-
5.
Yonemitsu T, Kawai N, Sato M, Tanihata H, Takasaka I, Nakai M, et al. Evaluation of transcatheter arterial embolization with gelatin sponge particles, microcoils, and n-butyl cyanoacrylate for acute arterial bleeding in a coagulopathic condition. J Vasc Interv Radiol. 2009;20(9):1176-87. [PubMed ID: 19643634]. https://doi.org/10.1016/j.jvir.2009.06.005.
-
6.
Stainsby D, MacLennan S, Hamilton PJ. Management of massive blood loss: a template guideline. Br J Anaesth. 2000;85(3):487-91. [PubMed ID: 11103199].
-
7.
Qiu L, He J, Ye X, Xie W, Shi J, Zheng W, et al. Invasive pulmonary fungal infection accompanied by severe hemoptysis in patients with hematologic diseases: a report of nine cases. Int J Hematol. 2009;90(1):108-12. [PubMed ID: 19484336]. https://doi.org/10.1007/s12185-009-0335-0.
-
8.
Veltri A, Anselmetti GC, Bartoli G, Martina MC, Regge D, Galli J, et al. Percutaneous treatment with amphotericin B of mycotic lung lesions from invasive aspergillosis: results in 10 immunocompromised patients. Eur Radiol. 2000;10(12):1939-44. [PubMed ID: 11305575]. https://doi.org/10.1007/s003300000469.
-
9.
Drooz AT, Lewis CA, Allen TE, Citron SJ, Cole PE, Freeman NJ, et al. Quality improvement guidelines for percutaneous transcatheter embolization. J Vasc Interv Radiol. 2003;14(9 Pt 2):S237-42. [PubMed ID: 14514825].
-
10.
Marshall TJ, Jackson JE. Vascular intervention in the thorax: bronchial artery embolization for haemoptysis. Eur Radiol. 1997;7(8):1221-7. [PubMed ID: 9377505]. https://doi.org/10.1007/s003300050279.
-
11.
Tonkin IL, Hanissian AS, Boulden TF, Baum SL, Gavant ML, Gold RE, et al. Bronchial arteriography and embolotherapy for hemoptysis in patients with cystic fibrosis. Cardiovasc Intervent Radiol. 1991;14(4):241-6. [PubMed ID: 1913738].
-
12.
Aina R, Oliva VL, Therasse E, Perreault P, Bui BT, Dufresne MP, et al. Arterial embolotherapy for upper gastrointestinal hemorrhage: Outcome assessment. J Vasc Interv Radiol. 2001;12:195-200.
-
13.
Breuer R, Lossos IS, Berkman N, Or R. Pulmonary complications of bone marrow transplantation. Respir Med. 1993;87(8):571-9. [PubMed ID: 8290740].
-
14.
Drew WL, Finley TN, Golde DW. Diagnostic lavage and occult pulmonary hemorrhage in thrombocytopenic immunocompromised patients. Am Rev Respir Dis. 1977;116(2):215-21. [PubMed ID: 889174]. https://doi.org/10.1164/arrd.1977.116.2.215.
-
15.
Ettinger NA, Trulock EP. Pulmonary considerations of organ transplantation. Part 2. Am Rev Respir Dis. 1991;144(1):213-23. [PubMed ID: 2064131]. https://doi.org/10.1164/ajrccm/144.1.213.
-
16.
Kahn FW, Jones JM, England DM. Diagnosis of pulmonary hemorrhage in the immunocompromised host. Am Rev Respir Dis. 1987;136(1):155-60. [PubMed ID: 3605828]. https://doi.org/10.1164/ajrccm/136.1.155.
-
17.
Singer C, Armstrong D, Rosen PP, Walzer PD, Yu B. Diffuse pulmonary infiltrates in immunosuppressed patients. Prospective study of 80 cases. Am J Med. 1979;66:110-20.
-
18.
Weisdorf DJ. Diffuse alveolar hemorrhage: an evolving problem? Leukemia. 2003;17(6):1049-50. [PubMed ID: 12764367]. https://doi.org/10.1038/sj.leu.2402921.
-
19.
Smith LJ, Katzenstein AL. Pathogenesis of massive pulmonary hemorrhage in acute leukemia. Arch Intern Med. 1982;142(12):2149-52. [PubMed ID: 6958217].