Abstract
Background:
We measure the umbilical cord thickness of newborn just after delivery with digital Vernier caliper and correlate the association with antenatal maternal risk factors. This helps the pediatricians to predict which newborn need close monitoring.Objectives:
The aim of indexed study to evaluate the umbilical cord thickness, maternal antenatal risk factors and determines the association between umbilical cord thickness and antenatal maternal risk factors.Methods:
This is a cross sectional prospective study conducted between 2020 and 2021 at Rajkiya Mahila Chikitsalaya, J L N Medical College, Ajmer, India. Total 303 newborn subjected for this study. Out of these 189 newborn enrolled as control group for this study. Enrolment of sample was simple as our convenience during the study period.Results:
Mean umbilical cord diameter was found smaller in newborns which associated with antenatal risk factors as compared to controls without antenatal risk factors (cases: 9.89 ± 2.53 mm; controls: 10.56 ± 2.26 mm) and the difference was statistically significant (P = 0.03). Oligohydramnios and meconium-stained liquor were found to be associated with the smaller umbilical cord diameter (P < 0.01).Conclusions:
Oligohydramnios and meconium-stained liquor were found to be significantly associated with the thin umbilical cord.Keywords
Antenatal Risk Factor Oligohydramnios Meconium Umbilical Cord Diameter Umbilical Cord Thickness
1. Background
Umbilical cord connects the fetus with placenta. It is a vital structure. Umbilical cord development began to start around third week of gestation and completely develop at seven weeks (1). Umbilical cord thickness depends on lumen of vessels and Wharton’s jelly (2, 3). The principle function of umbilical cord is keeping the cord vessels. These vessels exchange the blood between placenta and fetus and help in growth of fetus (4). The Umbilical cord diameter affects the overall outcome of fetus (5). Wharton's jelly is an extracellular matrix, gelatinous material. It protects the umbilical cord vessels from compression or bending (6). Intrauterine growth retardation (IUGR) predisposes by Reduction in wall thickness of the umbilical cord arteries and vein (7). Thus, the overall thickness of umbilical cord is contributed by its vessels, Wharton’s jelly. So, it can be concluded that reduction in umbilical cord thickness and diameter can compromises the fetal growth. Reduced fetal growth has its own implications on immediate postnatal and long-term neonatal outcomes.
Neonatal sepsis is the main causes of death in neonates. Various infections can be the origin of sepsis and infected umbilical cord is one of the sources of sepsis that can lead to cellulitis, omphalitis and eventually sepsis (8, 9). Bathing of neonates after birth does not affect the rate and type of infection of umbilical cord. But, early separation of cord was seen in non-bathing babies (10).
In spite of valuable effort of medical health services, there are various antenatal maternal risk factors affecting the health of newborn. Antenatal maternal risk factors are defined as maternal risk factors that adversely affecting the pregnancy and health of fetus are known as antenatal maternal risk factor. Severe anaemia, hypothyroidism, Gestational diabetes, Malpresentation, twin or multiple pregnancy, placenta previa, Bad obstetric history, hypertensive disorder of pregnancy are consider as high risk factors in pregnancy (11-14). Following antenatal maternal risk factors (Bad obstetric history, Blood transfusion during pregnancy, History of Pregnancy induced hypertension, Gestational diabetes mellitus, Hypothyroidism, Oligohydramnios, Meconium-stained liquor, Prolonged rupture of membrane, Malpresentation, Anaemia in mother) were studied and correlated with neonatal placental thickness (Table 1). So, the maternal first antenatal care visit completed by evaluation of maternal total cholesterol, high-density lipoprotein and low-density lipoprotein. These blood parameters could influence the outcome of umbilical cord length, diameter, and area (5).
Maternal Antenatal Risk Factors and Umbilical Cord Diameter a
| Parameters | Number of Neonates | Umbilical Corddiameter | P-Value |
|---|---|---|---|
| Bad obstetric history | 0.77 | ||
| Yes | 4 | 10.54125 ± 2.055425 | |
| No | 299 | 10.30832 ± 2.396631 | |
| Blood transfusion during pregnancy | 0.27 | ||
| Yes | 22 | 9.81364 ± 2.708103 | |
| No | 281 | 10.35036 ± 2.363914 | |
| History of pregnancy induced hypertension | 0.92 | ||
| Yes | 26 | 10.07442 ± 3.218658 | |
| No | 277 | 10.33364 ± 2.303087 | |
| Gestational diabetes mellitus | 0.39 | ||
| Yes | 5 | 9.55800 ± 1.965205 | |
| No | 298 | 10.32403 ± 2.396841 | |
| Hypothyroidism | 0.12 | ||
| Yes | 8 | 8.90063 ± 2.399012 | |
| No | 295 | 10.34965 ± 2.381842 | |
| Oligohydramnios | < 0.01 | ||
| Severe | 9 | 8.01389 ± 1.496234 | |
| Moderate | 7 | 9.35429 ± 1.830286 | |
| Mild | 7 | 9.23143 ± 2.571269 | |
| No | 280 | 10.43617 ± 2.380049 | |
| Meconium-stained liquor | < 0.01 | ||
| Yes | 26 | 8.864 ± 2.381 | |
| No | 277 | 10.445 ± 2.350 | |
| Prolonged rupture of membrane | 0.07 | ||
| Yes | 12 | 9.07208 ± 3.144532 | |
| No | 291 | 10.36250 ± 2.346379 | |
| Malpresentation | 0.67 | ||
| Yes | 14 | 10.37643 ± 2.337042 | |
| No | 289 | 10.30824 ± 2.396096 | |
| Anaemia in mother | 0.17 | ||
| Yes | 28 | 9.56179 ± 2.724864 | |
| No | 163 | 10.42900 ± 2.394953 |
2. Objectives
- To evaluate the umbilical cord thickness in newborn;
- To identified the antenatal maternal risk factors in study population;
- To determine the correlation between umbilical cord thickness and antenatal maternal risk factors.
3. Methods
3.1. Study Design
this is a cross sectional prospective study which was conducted in the neonatal intensive care unit of a tertiary care center in north India. This study conducted to collect data (umbilical cord thickness, antenatal history, after delivery follow-up of newborns). This study started from august 2020 to July 2021 at Rajkiya Mahila Chikitsalaya, Jawahar Lal Nehru Medical College, Ajmer, India.
3.2. Study Population
We enrolled 303 neonates for this study. One hundred and fourteen consecutive neonates born from mother completed gestation period 34 weeks and newborn birth weight > 1250 grams. Mother of These newborns had antenatal maternal risk factors. Mothers of other 189 newborns were free from antenatal maternal risk factors and considered as control group. In this group mother also completed gestation period 34 weeks and newborn birth weight > 1250 grams.
All patients were subjected to a protocol (as per proforma) which included a detailed clinical history of antenatal maternal risk factors, relevant examination, birth weight, Apgar score and meconium staining recorded. After delivery, umbilical cord diameter was measured for all neonates at 2.5 cm above the base of cord at neonatal side by digital vernier caliper (accuracy up to 0.01 mm) (Figure 1).
Measurement of umbilical cord thickness (diameter) with digital vernier caliper
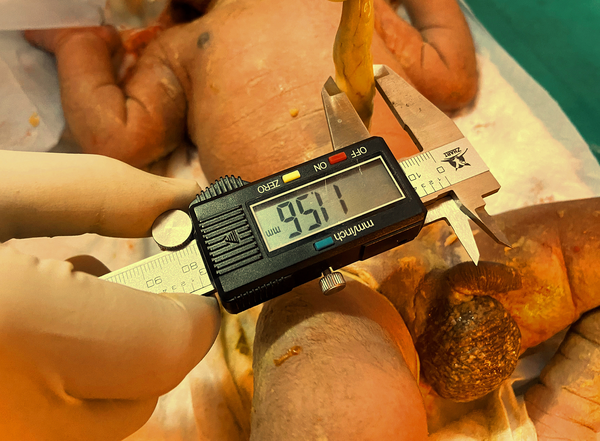
Digital vernier caliper used for this study – Brand – Q fun, material stainless steel.
3.3. Inclusion Criteria
- Neonates associated with antenatal risk factor was considered as case.
- Neonates who are not associated with antenatal risk factor was considered as control.
- Single pregnancy.
3.4. Exclusion Criteria
- Newborns having weight < 1250 gram;
- Newborn born before 34 weeks of Gestational age;
- Neonates with IUGR, low birth weight were excluded.
3.5. Study Size
We collect the sample as our convenience and simple.
3.6. Statistical Analysis
The data was analyzed using Statistical Package for Social Sciences (SPSS) Version 23. Proportions were compared using chi-square test while mean values were compared using Independent‘t’ test. Multiple linear regression analysis was done to see the association of different risk factors with umbilical cord diameters. The confidence limit of the study was kept at 95% hence a P-value less than 0.05 was considered statistically significant.
4. Results
Three hundred three newborns were found suitable according to inclusion criteria during study period. We found mean gestation age was 37 ± 2.1 weeks in the study population. Mean gestational age at delivery was significantly lower in mother with antenatal risk factor as compared to those without antenatal risk factors (37 ± 2.1 week versus 38.14 ± 1.64 week; P = 0.01). There was preponderance of male newborn in cases (55%) while gender distribution in controls was equal. Mean umbilical cord diameter was smaller in newborns with antenatal risk factors as compared to newborns without antenatal risk factors (cases: 9.89 ± 2.53 mm; controls: 10.56 ± 2.26 mm) and the difference was statistically significant (P = 0.03).
Oligohydramnios and meconium-stained liquor were found to be associated with the smaller umbilical cord diameter (P < 0.01) (Table 1). With increase in severity of oligohydramnios from mild to moderate to severe, the umbilical cord diameter was found to be significantly smaller (P < 0.01). There was no significant correlation between maternal age and gestation with umbilical cord diameter (Table 2).
Correlation Between Maternal Parameters and Umbilical Cord Diameter
| Variables | Values |
|---|---|
| Maternal age | |
| r-value | 0.016 |
| P-value | 0.804 |
| No. | 245 |
| Gestation | |
| r-value | 0.081 |
| P-value | 0.159 |
| No. | 303 |
The multivariate linear regression analysis of umbilical cord diameter with oligohydramnios and meconium-stained liquor were found significant association than other variants (Table 3).
Multivariate Linear Regression Analysis to See the Association of Different Factors with Umbilical Cord Diameter
| Unstandardized Coefficients | t | P-Value | 95.0% Confidence Interval for B | |||
|---|---|---|---|---|---|---|
| B | Std. Error | Lower Bound | Upper Bound | |||
| Oligohydramnios | -0.541 | 0.227 | -2.386 | 0.018 | -0.986 | -0.095 |
| Meconium stained liquor | -1.193 | 0.484 | -2.464 | 0.014 | -2.145 | -0.240 |
Our study showed that 127 newborns were admitted and 176 newborns not required admission (Table 4). Total 15 newborns were expired, all these were associated with antenatal maternal risk factors and less thickness of umbilical cord (Table 4).
Neonatal Outcome with Relation to Umbilical Cord Diameters
| Need Admission | No. | Umbilical Cord Diameter, Mean ± SD | P-Value |
|---|---|---|---|
| Outcome | 0.001 | ||
| Admitted | |||
| Expired | 15 | 9.96700 ± 2.952235 | |
| Discharge | 112 | 9.65420 ± 2.474955 | |
| Not admitted | |||
| Shift | 176 | 10.75896 ± 2.187243 |
5. Discussion
This prospective observational study was conducted to evaluate the association between umbilical cord diameter and maternal antenatal risk factors. This study indicates that umbilical cord diameter is the indictor of health status of newborn. A wide diameter of cord indicates a good health of newborn. Udoh et al., Lee et al. and Tahmasebi and Alighanbari also reported similar results in their study (15-17). The only limitations of these studies were ultrasound guided measurement of cord thickness. Ultrasound measured diameter may have observational bias. Manual measurement of cord thickness is the strength of our study.
In a previously reported study about measurement of umbilical cord thickness after 20 weeks of gestation, they measured thickness by USG guided. They concluded that umbilical cord thickness provide us information about adverse pregnancy outcome (15). We agree with this concept. We measure umbilical cord thickness just after delivery and found newborn with smaller cord thickness need intensive care. Few newborn expired even after intensive care (15).
Newborns with small umbilical cord diameter got admitted for intensive care compare to newborns shifted to mother without need of admission in our study. This is supported by previous study that stat umbilical cord thickness affects the fetal outcome. Umbilical cord thickness can cause low birth weight baby, and adverse pregnancy outcome (18).
Difference in diameter of umbilical cord between male and female neonates was not found significant in this study. This is in contrast with previous studies which all reported thick umbilical cord in males (17, 19-22).
Oligohydramnios is defined as < 5 amniotic fluid index (AFI) (23). Oligohydramnios is the indicator poor outcome of pregnancy and fetal health (24-26). Our study also agreed with this. Eight newborn delivered from mother containing oligohydramnios. All these babies were admitted in intensive care unit.
Many antenatal risk factors were evaluated for its effect on umbilical cord diameter. Oligohydramnios and meconium-stained liquor were found to be associated with the smaller umbilical cord diameter. Our study confirms that umbilical cord thickness strongly associated with meconium stained liquor. Similar result also found in Tahmasebi and Alighanbari study (15).
Comprehensive inclusion of antenatal risk factors and measurement of umbilical cord diameter by digital caliper are strengths of this study while non-inclusion of preterm neonates, not doing serial assessment of fetal umbilical cord diameter by using ultrasonography are some of the limitations.
5.1. Conclusions
Presence of antenatal risk factors leads to thin umbilical cord. This suggests that thin umbilical cord diameter may contribute to the spectrum of placental insufficiency leading to fetal growth restriction and its implication on the neonatal outcome. Thin umbilical cord has been found to be significantly associated with oligohydramnios and meconium-stained liquor. More severe is the oligohydramnios, thinner is the umbilical cord. However no significant correlation was demonstrated between umbilical cord thickness and maternal age and gestation. A large series is needed to confirm these findings and its implication on neonatal outcome.
References
-
1.
Heil JR, Bordoni B. Embryology, Umbilical Cord. Treasure Island, USA: Stat Pearls Publishing; 2022.
-
2.
Weissman A, Jakobi P, Bronshtein M, Goldstein I. Sonographic measurements of the umbilical cord and vessels during normal pregnancies. J Ultrasound Med. 1994;13(1):11-4. [PubMed ID: 7636947]. https://doi.org/10.7863/jum.1994.13.1.11.
-
3.
Predanic M, Perni SC, Chasen ST. The umbilical cord thickness measured at 18-23 weeks of gestational age. J Matern Fetal Neonatal Med. 2005;17(2):111-6. [PubMed ID: 16076617]. https://doi.org/10.1080/14767050500042824.
-
4.
Moshiri M, Zaidi SF, Robinson TJ, Bhargava P, Siebert JR, Dubinsky TJ, et al. Comprehensive imaging review of abnormalities of the umbilical cord. Radiographics. 2014;34(1):179-96. [PubMed ID: 24428290]. https://doi.org/10.1148/rg.341125127.
-
5.
Bimpong S, Abaidoo CS, Tetteh J, Okwan D. Maternal first antenatal care visit biometric indices as potential predictors of umbilical cord morphometric parameters. J Neonatal Perinatal Med. 2022;15(1):129-36. [PubMed ID: 34151869]. https://doi.org/10.3233/NPM-210734.
-
6.
Raio L, Ghezzi F, Di Naro E, Franchi M, Maymon E, Mueller MD, et al. Prenatal diagnosis of a lean umbilical cord: a simple marker for the fetus at risk of being small for gestational age at birth. Ultrasound Obstet Gynecol. 1999;13(3):176-80. [PubMed ID: 10204208]. https://doi.org/10.1046/j.1469-0705.1999.13030176.x.
-
7.
Scott JM, Wilkinson R. Further studies on the umbilical cord and its water content. J Clin Pathol. 1978;31(10):944-8. [PubMed ID: 711902]. [PubMed Central ID: PMC1145457]. https://doi.org/10.1136/jcp.31.10.944.
-
8.
Coffey PS, Brown SC. Umbilical cord-care practices in low- and middle-income countries: a systematic review. BMC Pregnancy Childbirth. 2017;17(1):68. [PubMed ID: 28219420]. [PubMed Central ID: PMC5319165]. https://doi.org/10.1186/s12884-017-1250-7.
-
9.
Newborns: reducing mortality. World Health Organization; 2019, [cited 6/19/2019]. Available from: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight.
-
10.
Siroosbakht S, Aarabi N, Rezakhaniha B. Bathing or Not Bathing: Which Is Better for Umbilical Cord Separation Time and Bacterial Colonization in Neonates? Arch Pediatr Infect Dis. 2020;9(2). https://doi.org/10.5812/pedinfect.104100.
-
11.
Ayano B. Assessment of Prevalence and Risk Factors for Anemia Among Pregnant Mothers Attending Anc Clinic at Adama Hospital Medical Collage, Adama, Ethiopia, 2017. J Obstet Gynaecol. 2018;6(3):31-9. https://doi.org/10.11648/j.jgo.20180603.11.
-
12.
Chemir F, Alemseged F, Workneh D. Satisfaction with focused antenatal care service and associated factors among pregnant women attending focused antenatal care at health centers in Jimma town, Jimma zone, South West Ethiopia; a facility based cross-sectional study triangulated with qualitative study. BMC Res Notes. 2014;7:164. [PubMed ID: 24646407]. [PubMed Central ID: PMC3994781]. https://doi.org/10.1186/1756-0500-7-164.
-
13.
Majella MG, Sarveswaran G, Krishnamoorthy Y, Sivaranjini K, Arikrishnan K, Kumar SG. A longitudinal study on high risk pregnancy and its outcome among antenatal women attending rural primary health centre in Puducherry, South India. J Educ Health Promot. 2019;8:12. [PubMed ID: 30815483]. [PubMed Central ID: PMC6378831]. https://doi.org/10.4103/jehp.jehp_144_18.
-
14.
K. C J, D P, A G. Prevalence of high risk among pregnant women attending antenatal clinic in rural field practice area of Jawaharlal Nehru Medical College, Belgavi, Karnataka, India. Int J Community Med Public Health. 2017;4(4):1257-9. https://doi.org/10.18203/2394-6040.ijcmph20171359.
-
15.
Tahmasebi M, Alighanbari R. Evaluation of umbilical cord thickness, cross-sectional area, and coiling index as predictors of pregnancy outcome. Indian J Radiol Imaging. 2011;21(3):195-8. [PubMed ID: 22013294]. [PubMed Central ID: PMC3190491]. https://doi.org/10.4103/0971-3026.85367.
-
16.
Udoh BE, Erim A, Anthony E. Sonographic Assessment of Umbilical Cord Diameter as an Indicator of Fetal Growth and Perinatal Outcome. J Diagn Med Sonogr. 2020;37(1):41-5. https://doi.org/10.1177/8756479320963041.
-
17.
Lee SM, Kim DY, Cho S, Noh SM, Park HL, Lee G. Correlations between the Status of the Umbilical Cord and Neonatal Health Status. Child Health Nurs Res. 2020;26(3):348-56. [PubMed ID: 35004478]. [PubMed Central ID: PMC8650973]. https://doi.org/10.4094/chnr.2020.26.3.348.
-
18.
Chakrabarti M, Ray SD, Roy D, Ghosh RM, Agarwal A. Correlation of Umbilical Cord Thickness with Fetal Birth Weight - a Prospective Study in Rural Bengal. J Evol Med Dent Sci. 2015;4(76):13170-3. https://doi.org/10.14260/jemds/2015/1896.
-
19.
Elghazaly EA, Al Awad K, Alghamdi J. Correlation between the Measurement of the Umbilical Cord Diameter and the Birth Weight Outcome, in Sudanese Neonates. Int J Health Sci Res. 2018;8(11):97-101.
-
20.
Sepulveda W, Alcalde JL, Schnapp C, Bravo M. Perinatal outcome after prenatal diagnosis of placental chorioangioma. Obstet Gynecol. 2003;102(5 Pt 1):1028-33. [PubMed ID: 14672481]. https://doi.org/10.1016/s0029-7844(03)00859-7.
-
21.
Barbieri C, Cecatti JG, Surita FG, Costa ML, Marussi EF, Costa JV. Area of Wharton's jelly as an estimate of the thickness of the umbilical cord and its relationship with estimated fetal weight. Reprod Health. 2011;8:1186-8. [PubMed ID: 22054163]. [PubMed Central ID: PMC3219549]. https://doi.org/10.1186/1742-4755-8-32.
-
22.
Soysal C, Şişman Hİ, Bıyık İ, Erten Ö, Deliloğlu B, Geçkalan Soysal D, et al. The relationship between umbilical cord measurements and newborn outcomes. Perinatal Journal. 2021;29(3):225-30. https://doi.org/10.2399/prn.21.0293008.
-
23.
Phelan JP, Smith CV, Broussard P, Small M. Amniotic fluid volume assessment with the four-quadrant technique at 36-42 weeks' gestation. J Reprod Med. 1987;32(7):540-2. [PubMed ID: 3305930].
-
24.
Gibbs RS, Danforth DN, Karlan BY, Haney AF. Danforth's Obstetrics and Gynecology. 10th ed. New York, USA: Lippincott Williams & Wilkins; 2008.
-
25.
Cunningham FG, Williams JW, Leveno KJ, Bloom S, Hauth JC. Williams Obstetrics. 23rd ed. New York, USA: McGraw-Hill Medical; 2009.
-
26.
Ghimire S, Ghimire A, Chapagain S, Paudel S. Pregnancy outcome in cases of oligohydramnios after 28 weeks of gestation. Int J Adv Med Health Res. 2016;3(2):68-72. https://doi.org/10.4103/2349-4220.195939.