1. Background
Cystic fibrosis (CF) is an autosomal recessive, multisystem, life-threatening disorder affecting more than 30 000 people in the United States and 70 000 worldwide (1, 2). Over the years, CF has been reported in Asian countries (3).
This disease results from a mutation in the CF transmembrane conductance regulator (CFTR) gene (4, 5). The CFTR gene-encoded protein is responsible for transporting chloride and bicarbonate anions through the plasma membrane. CF transmembrane conductance regulator is commonly expressed in epithelial cells that line the mucous membranes and submucosal glands of the airways. Dysfunctional CFTR leads to inflammation and infection of the airways, as well as a progressive decrease in lung function, which leads to respiratory failure and premature death (5-7). There are many determinants of pulmonary function in CF, including underlying genetic defects, existing bacterial pathogens, medication adherence, psychosocial factors, and nutritional status (8, 9). The disease also affects various systems of the body, including the gastrointestinal tract, pancreas, sweat ducts, skeletal muscle, cardiovascular, and reproductive organs (6, 10). Involvement of the gastrointestinal tract causes inefficient absorption of nutrients. This leads to mineral and vitamin deficiencies, causing a lack of proper growth and worsening the disease (11, 12).
Pancreatic insufficiency and its resulting fat malabsorption occur in approximately 85% of patients with CF and puts patients at risk for severe deficiency of fat-soluble vitamins (A, D, E, and K) and altered homeostasis of trace elements, such as calcium, magnesium, iron, copper, selenium, and zinc (1, 13). Therefore, deficiencies in these vitamins and elements seem to be common in CF patients. However, there is no consensus on the serum level of minerals and vitamins in these patients. Therefore, it is important to compensate for nutritional deficiencies in CF patients to increase their quality of life. Since the dietary supplements used for these patients are not prescribed according to a specific and regulated diet, it is necessary to conduct studies in this field.
2. Objectives
This study aimed to determine the relationship between magnesium, copper, zinc, selenium, complete blood cell count (CBC), and vitamin D levels with disease severity in patients with CF patients referred to Mofid Children’s Hospital in Tehran, Iran.
3. Methods
3.1. Study Design and Sample
In this cross-sectional study, children with CF referred to the pulmonary subspecialty clinic of Mofid Children’s Hospital in 2017 were included. Before participating, the objectives of the study were carefully explained to patients and their parents, and informed consent was obtained. Inclusion criteria were children with a confirmed diagnosis of CF by a sweat test and/or genetic testing, age of ≤ 18 years, and not taking supplements before the tests. To calculate the sample size, based on α = 0.05, P = 40%, d = 0.1, and a power of 90%, 90 people were estimated, of whom 84 completed the study.
3.2. Data Recruitment
Demographic and anthropometric data of patients were collected through a researcher-made checklist. The severity of pulmonary involvement was measured based on chest X-ray findings. If the patient did not have a radiograph in the past 3 months, a new CXR was taken, and the severity of the disease was determined according to the Brasfield scoring system (BSS). The score is based on radiologic findings regarding air trapping, linear markings, nodular cystic lesions, large lesions, and general severity (14-17).
A blood sample (2 mL) was taken from each patient. The serum levels of magnesium, copper, zinc, selenium, CBC, and vitamin D were determined. The blood levels of copper, magnesium, and zinc were measured by a calorimetric method using an autoanalyzer, calcium, phosphorus, and serum alkaline phosphate by a Biolis 24i method using a premium device, CBC by a Sysmex cell counter device, serum selenium level using an HPLC device and automatic absorption, and vitamin D by enzyme-linked immunosorbent assay (ELISA).
3.3. Statistical Analysis
Descriptive statistics were reported for all variables. Continuous data are presented as mean ± SD, and categorical data are presented as percentages. Pearson correlation coefficient, t test, χ2 test, or Fisher exact test was used for analytical analysis. For all tests, a P value of less than 0.05 was considered statistically significant. Statistical analyses were performed using SPSS version 24.0 (SPSS Inc, Chicago, Ill, USA).
4. Results
4.1. Demographic Data
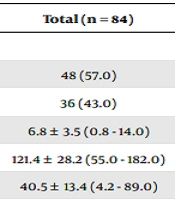
This study was performed on 84 children with CF. The mean age of the participants was 6.8 ± 3.5 years (range, 0.8 - 14 years), and the mean age of the diagnosis was 1.2 ± 2.4 years (range, 0.08 - 10 years). Thirty patients (43%) were girls, and 48 (57%) were boys.
The mean z score in the patients was -0.63 ± 2.0 (range, -8.0 to 5.0). The z score findings showed that 26 patients (31%) had normal weight, 5 (6%) were obese, 18 (21%) had mild malnutrition, 17 (21%) had moderate malnutrition, and 18 (21%) had severe malnutrition. More detailed characteristics of the patients are presented in Table 1.
| Demographics | Total (n = 84) |
|---|---|
| Sex | |
| Boys | 48 (57.0) |
| Girls | 36 (43.0) |
| Age | 6.8 ± 3.5 (0.8 - 14.0) |
| Height | 121.4 ± 28.2 (55.0 - 182.0) |
| Weight | 40.5 ± 13.4 (4.2 - 89.0) |
| Body mass index | 16.4 ± 5.0 (2.6 - 48.5) |
| Disease-related specs | |
| Family history of CF | 12 (14.3) |
| Age at diagnosis | 1.2 ± 2.4 (0.08 - 10.0) |
| Malnutrition | 53 (63.1) |
| Exercise intolerance and breathlessness | 17 (20.2) |
| Sputum culture | |
| Negative | 29 (34.5) |
| Burkholderia | 1 (1.2) |
| Pseudomonas | 54 (64.3) |
| The severity of pulmonary involvement | |
| Mild | 25 (30.0) |
| Moderate | 39 (46.0) |
| Severe | 20 (24.0) |
Abbreviation: CF, cystic fibrosis.
a Values are expressed as count (%) and mean ± SD (range).
4.2. Pulmonary Involvement
The mean Brasfield score in patients was 16.89 ± 4.45 (range, 4.0 - 24.0). Based on BSS, the severity of pulmonary involvement was mild in 25 patients (30%), moderate in 39 (46%), and severe in 20 (24%). The most common sputum culture was Pseudomonas (64.3%).
4.3. Serum Analysis
The highest percentage of deficiency was related to vitamin D (56%), zinc (38%), copper (32.1%), and selenium (21.4%). The percentage of selenium deficiency was 18%. Moreover, 10.7% of patients had hemoglobin levels lower than normal, and none of them had leukopenia or thrombocytopenia. The detailed distribution of serum analysis findings is presented in Table 2.
| Mean ± SD (Range) | Normal Range | Patients with Values Less Than Normal No. (%) | Patients with Values More Than Normal No. (%) | |
|---|---|---|---|---|
| CBC | ||||
| White blood cell | 8453.6 ± 2674.7 (4300 - 17300) | 5000 - 10000 | - | 17 (20.2) |
| Neutrophils | 46.4 ± 11.6 (17 - 73) | 50% - 70% | 55 (65.4) | 2 (2.3) |
| Lymphocytes | 46.8 ± 11.2 (16 - 80) | 30% - 45% | 5 (5.9) | 46 (54.7) |
| Eosinophils | 2.5 ± 1.7 (0 - 8) | 0% - 4% | - | 11 (13.0) |
| Hematocrit | 40.1 ± 3.7 (27 - 49.8) | 37% - 50% | 15 (17.8) | - |
| Hemoglobin (g/dL) | 13.6 ± 1.5 (8.4 - 17.9) | 12 - 16 | 9 (10.7) | 5 (5.9) |
| Platelets (mg/dL) | 292881 ± 85728 (161000 - 713000) | 150000 - 400000 | - | 9 (10.7) |
| Calcium (g/dL) | 9.4 ± 0.5 (7.9 - 10.5) | 8.6 - 10.2 | 2 (2.3) | 5 (5.9) |
| Phosphorus (g/dL) | 4.5 ± 0.7 (3.0 - 6.1) | 3.0 - 4.5 | - | 50 (59.5) |
| Zinc (μg/dL) | 82.8 ± 15.5 (67.0 - 140.0) | 75 - 140 | 32 (38.0) | - |
| Copper (μg/dL) | 115.0 ± 24.1 (71.0 - 189.0) | 100 - 200 | 27 (32.1) | - |
| Magnesium (μg/dL) | 2.0 ± 0.14 (1.6 - 2.4) | 1.6 - 2.6 | - | - |
| Selenium (ng/mL) | 66.5 ± 34.1 (13.0 - 202.0) | 46 - 143 | 18 (21.4) | 4 (4.7) |
| Vitamin D (ng/mL) | 32.8 ± 16.1 (4.5 - 92.0) | 30 - 60 | 47 (56.0) | 6 (7.1) |
Abbreviation: CBC, complete blood cell.
4.4. The Relationship Between Serum Analysis and Pulmonary Involvement
The results of serum analysis showed no statistically significant relationship between the severity of pulmonary involvement (according to the Brasfield score) and hypocalcemia (P = 0.93 and r = 0.009), hypophosphatemia (P = 0.13 and r = -0.166), zinc deficiency (P = 0.07 and r = -0.196), and vitamin D levels (P = 0.08 and r = -0.191) (Table 3). However, there was a statistically significant relationship between the severity of pulmonary involvement and copper (P = 0.007 and r = -0.292) and selenium deficiency (P < 0.001 and r = -0.418). There was a statistically significant relationship between malnutrition and serum vitamin D levels (P = 0.03), but there was no statistically significant relationship between malnutrition and serum selenium levels (P = 0.67).
| Elements | P Value (Deficiencies and Pulmonary Involvement) | r (Levels and Pulmonary Involvement) |
|---|---|---|
| Calcium | 0.93 | 0.009 |
| Phosphate | 0.13 | -0.166 |
| Zinc | 0.07 | -0.196 |
| Vitamin D | 0.08 | -0.191 |
| Copper | 0.007 | -0.292 |
| Selenium | < 0.001 | -0.418 |
5. Discussion
The present study was performed to investigate the relationship between disease severity and magnesium, copper, zinc, selenium, CBC, and vitamin D levels in CF patients. According to BSS, the severity of lung disease was mild (30%), moderate (46%), and severe (24%), and the highest percentage of deficiency was related to vitamin D. However, there was no statistically significant relationship between the severity of pulmonary involvement and vitamin D levels. In contrast, serum copper and selenium levels were negatively associated with the severity of pulmonary involvement in CF patients.
In support of this study, Dara et al. assessed 78 CF children, of whom 21 (26%) had mild malnutrition, 26 (34%) had moderate malnutrition, and 31 (40%) had severe malnutrition (18). These results show that nutritional interventions are needed for CF patients.
The mean Brasfield score could vary in different study settings. For example, a study measuring pulmonary function in patients with CF showed that the mean Brasfield score ranged from 20.2 to 22.3 in children under 1 to 5 years old (19, 20). This point highlights the imperative to delve into possible explanations behind the differences in the severity of pulmonary involvement in CF patients, such as nutritional status.
In contrast with our study, Sexauer et al. studied 597 patients with a mean age of 22.2 ± 11.9 years, observing a significant relationship between serum levels of vitamin D and pulmonary function in CF patients (9). In addition, a study in Pakistan showed that 69 CF patients were deficient in vitamin D. Vitamin D deficiency was also associated with an increase in the number of annual pulmonary exacerbations and Pseudomonas infections (21). Moreover, Vanstone et al. indicated that depleted vitamin D status was a significant cause of developing pulmonary exacerbations (22). Future studies are required to justify this contrast in the findings.
In this study, 38% of the subjects were zinc deficient, and no statistically significant relationship was observed between the severity of pulmonary involvement and serum zinc deficiency. In another study in Spain, 17.6% of CF patients had low zinc levels, and 23.5% had dietary zinc deficiency (23). Safai-Kutti et al., in a placebo-controlled double-blind crossover study on children with CF, showed that all subjects initially had low plasma zinc levels. They reported no change in clinical status, lung function, or growth rate in response to zinc or placebo (24). Another study reported that serum zinc levels in children with CF were similar to serum zinc levels in group controls, but children with lower serum zinc levels had significantly worse results in pulmonary function tests (25).
Copper is an essential trace metal that acts as a cofactor for various enzymes. Studies have shown low copper enzyme activity in patients with CF, indicating abnormal copper homeostasis and metabolism as the cause of moderate copper deficiency (26). A study in north India showed that 44% of CF cases were copper deficient and had lower levels of copper during periods of exacerbation (13). Another study showed no significant difference in serum copper when comparing CF patients with different respiratory and pancreatic functions and disease severity. However, mean serum copper was significantly lower in the undernourished than in the eutrophic CF patients (27). Hence, the role of copper deficiency in the severity of CF disease needs to be further assessed.
Regarding selenium, there are reports of lower plasma selenium, selenocysteine, and other selenium compounds in patients with CF compared with healthy controls (28, 29). Similar to our findings, a study of 46 CF patients found that elevated plasma selenium levels improved lung function and were positively correlated (30).
In this study, no statistically significant relationship was observed between the severity of pulmonary involvement and the distribution of CBC values. In a case-control study, the number of neutrophils, monocytes, and total white blood cells in the CF group was higher than in the control group (31).
All in all, nutrition is one of the several factors affecting the prognosis of CF. Thus, after being established, the role of various nutritional supplements could be included in CF management guidelines for the use of pediatricians. More importantly, parents’ consultation and raising their awareness seem essential regarding these supplements.
Limitations of the study include its cross-sectional and observational design and being recruited from only 1 clinic. As mentioned above, the findings of this study need to be further evaluated by nutritional interventions in case-control studies with a control group of healthy children. The strengths of this study include the simultaneous investigation of different elements and CBC values for any possible associations with the severity of pulmonary involvement in CF patients.
5.1. Conclusions
The nutritional status might affect the pulmonary involvement and the severity of the disease. However, further interventional studies are required to investigate the possible causal relationships. It seems essential to have a proper diet and use nutritional supplements and vitamins in these patients. Furthermore, copper and selenium levels would better be measured regularly to prevent severe diseases in these patients. Finally, due to the high prevalence of vitamin D deficiency in patients and its relationship with malnutrition, it is necessary to take periodic measurements in these patients and, if necessary, appropriate treatment measures.