Abstract
Background:
Soft corals of the genus Sinularia are well recognized as a rich source of steroidal compounds. These constituents have been reported as possessing antitumor, antimicrobial, and antiviral activities.Objectives:
This study was designed to isolate and elucidate antibacterial and cytotoxic compounds from the soft coral Sinularia polydactila.Methods:
Structure elucidation of steroids was determined based on spectroscopic data through 1D and 2D NMR analyses and mass spectrometry, with the results compared to data in the literature. Antibacterial activity was determined using four human bacterial pathogens, namely B. subtilis (ATCC 6633), P. aeruginosa (ATCC 27853), S. aureus (ATCC 25923), and E. coli (ATCC 25922). Cytotoxic activity was evaluated using the human colon cancer cell HCT 116 and brine shrimp lethality assay (BSLA).Results:
Two steroids (Compounds 1 - 2) were isolated from the Indonesian soft coral Sinularia polydactila. (22R,23R,24R)-22,23-methylene-24-methylcholest-6-en-5α,8α-epidioxy-3β-ol (1) and 5α,8α-Epidioxy-24(R)-methylcholesta-6,22-dien-3α-ol (2) showed moderate activity against colon carcinoma cancer HCT 116 at the concentrations of 24.8 and 27.3 μg/mL, respectively. Furthermore, compounds 1 and 2 showed cytotoxic activity using the brine shrimp lethality assay with the concentrations of 57.1 and 121.3 3 μg/mL, respectively. Compound 2 showed moderate activity against S. aureus and B. subtilis at the 250 μg/mL concentration.Conclusions:
Two steroids isolated from soft coral Sinularia polydactila were found to possess moderate cytotoxic and antibacterial activities.Keywords
Steroid Sinularia polydactila Cytotoxic Antibacterial Indonesia
1. Background
Soft corals related to the genus Sinularia, comprising almost 90 species, are broadly distributed in the tropical marine environment (1). A highly diverse group of marine organisms are found on coral reefs, which are known to produce a wide variety of bioactive compounds (2, 3). These compounds, which are significant in the chemical ecology of organisms, possess a range of biological characteristics such as antitumor, antimicrobial, and antiviral activities (4-6). Numerous newly isolated secondary metabolites have unique chemical structures, including sesquiterpenes, diterpenes, and steroids (3). Many of them are seen as promising sources of new antibacterial and anticancer agents.
Steroids from the genus Sinularia have been known for their cytotoxic activity against several cancer cell lines. For example, ergosta-3β,5α,6β-triol exhibits significant cytotoxic effect against lung carcinoma (A-549), pancreatic epithelioid carcinoma (PANC-1), and cervical adenocarcinoma (HeLa) cells with the IC50 values of 27.12, 20.51, and 24.64 μM, respectively (7). Sinubrassione and Ergosta-1β,3β,5α,6β-tetraol show significant activity against pancreatic epithelioid carcinoma (PANC-1) (8). Furthermore, ergosta-1α,3β,5α,6β,11α-pentaol inhibit the growth of HeLa cells and pregnedioside A against A-549 cell lines. Moreover, several steroids also exhibit antibacterial activity (8). For example, 3β,5α,6β)-ergost-24(28)-en-3,5,6,19-tetrol 19-monoacetate shows considerable antibacterial activity against Staphylococcus aureus with the MIC of 15.6 μM (9).
Indonesia is an archipelagic country that consists of more than 17,500 islands and has a long coastline of 81.000 km. This geographical situation has made Indonesia the world’s largest archipelagic and mega biodiverse region in the world. Indonesian marine biodiversity offers an excellent opportunity to produce secondary metabolites with unusual chemical characteristics. These metabolites often show pharmacological activities that are important for producing drug candidates, such as anticancer agents. In continuing our research program aimed to search secondary metabolites from Indonesian marine invertebrates, we recently studied the chemical composition of Indonesian marine invertebrates, such as sponges, soft corals, and ascidians (10-12). In this context, we analyzed a specimen of the soft coral Sinularia polydactila, which was collected at Lembeh strait in North Sulawesi, Indonesia. From the soft coral Sinularia polydactila extract, we isolated two known compounds identified as (22R,23R,24R)-22,23-methylene-24-methylcholest-6-en-5α,8α-epidioxy-3β-ol (1) and 5α,8α-Epidioxy-24(R)-methylcholesta-6,22-dien-3α-ol (2).
2. Objectives
In our research for pharmacological activities from Indonesian marine invertebrates, we examined the soft coral Sinularia polydactila. The present work focused on the isolation, purification, identification of bioactive compounds, and cytotoxic and antibacterial properties of this soft coral.
3. Methods
3.1. General Experimental Procedures
All the organic solvents, including Silica gel 60 and silica gel coated F254, were purchased from Merck. JEOL NMR spectrometer was used to measure 1H (500 MHz) and 13C (125 MHz) NMR. The residual solvent signal (CDCl3: δH 7.26, δC 77.0) was used as the chemical shift reference. Hitachi Chromaster HPLC system equipped with UV/VIS detector and Hypersil ODS C18 Column (Thermo Fischer) was used to isolate and purify the compounds.
3.2. Biological Material
The scuba diving technique was performed to collect the soft coral Sinularia polydactila at a depth of 8 m from Lembeh strait, Indonesia, in November 2016. It was transported to the laboratory in an ice container box and then stored at -20°C until extraction. A voucher specimen has been deposited at the Research Center for Biotechnology, Indonesian Institute of Sciences (LIPI), with the sample code LBH005.
3.3. Extraction and Isolation
To obtain a crude extract, the collected (500 g, wet/weight) samples were lyophilized and cut into small pieces and then extracted with MeOH: CH2Cl2 (1:1) at room temperature for 24 h. The extract (20 g) was put through column chromatography and eluted based on non-polar to polar solvents gradient. Antibacterial and cytotoxic activities are shown in n-hexane: ethyl acetate (4:6) fraction (19 mg). Furthermore, this fraction was purified by HPLC with UV-Vis wavelength at 210 nm (MeOH/H2O, 95:5) using a Hypersil ODS C18 column to afford 1 (1.3 mg) and 2 (1.4 mg) (Figure 1). The structures of the secondary metabolites were figured by NMR and LC/MS spectroscopy and compared with the literature.
Chemical structure of polyhydroxylated steroids
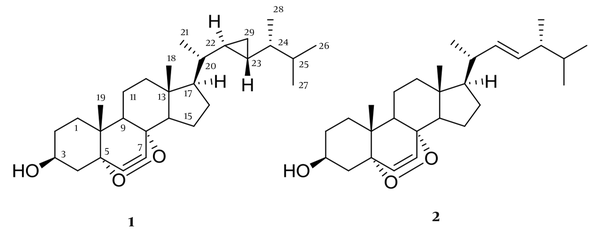
Compound 1 was identified as (22R,23R,24R)-22,23-methylene-24-methylcholest-6-en-5α,8α-epidioxy-3β-ol, suggesting the molecular formula C29H46O3, with 7 degrees of unsaturation, which produced a molecular ion at m/z 465.34 [M+Na]+, suggesting the molecular formula. 1H-NMR (500 MHz, CDCl3, J in Hz); δ 0.14 (2H, m, H-29), 0.30 (1H, m, H-22), 0.53 (1H, m, H-23), 0.54 (1H, m, H-24), 0.76 (3H, s, H-18), 0.85 (3H, d, J = 6.9 Hz, H-28), 0.89 (3H, d, J = 6.9 Hz, H-27), 0.90 (3H, s, H-19), 0.91 (6H, d, J = 6.3 Hz, H-21), 0.91 (6H, d, J = 6.3 Hz, H-26), 3.98 (1H, m, H-3), 6.24 (1H, d, J = 8.4 Hz, H-6), 6.51 (1H, d, J = 8.4 Hz, H-7). 13C NMR (CDCl3, 125 MHz): 38.8 (C-1), 30.5 (C-2), 66.3 (C-3), 50.8 (C-4), 78.8 (C-5), 130.8 (C-6), 135.4 (C-7), 80.2 (C-8), 34.8 (C-9), 36.3 (C-10), 21.1 (C-11), 38.6 (C-12), 45.8 (C-13), 50.7 (C-14), 28.4 (C-15), 24.4 (C-16), 58.4 (C-17), 12.9 (C-18), 18.1 (C-19), 40.5 (C-20), 19.4 (C-21), 23.2 (C-22), 24.9 (C-23), 45.3 (C-24), 32.4 (C-25), 18.5 (C-26), 20.7 (C-27), 14.7 (C-28), 10.3 (C-29).
Compound 2 was identified as 5α,8α-Epidioxy-24(R)-methylcholesta-6,22-dien-3α-ol. The ESIMS showed peak m/z 461.54 [M+Na]+, suggesting the molecular formula C28H44O31H-NMR (500 MHz, CDCI3), 6.51 (1H, d, J = 8.4 Hz, H-7), 6.24 (1H, d, J = 8.4 Hz, H-6), 5.22 (1H, d, J = 15.1 Hz, H-22), 5.13 (1H, d, J = 15.1 Hz, H-23), 3.97 (1H, m, H-3), 1,01 (3H, d, J = 6.4 Hz, H-21), 0.91 (3H, d, J = 7.2 Hz, H-28), 0.88 (3H, s, H-19), 0.82 (3H, d, J = 6.6 Hz, H-26), 0.84 (3H, s, H-18), 0.81 (3H, d, J = 6.6 Hz, H-27). 13C NMR (CDCl3, 125 MHz): 35.1(C-1), 32.3 (C-2), 66.8 (C-3), 36.8 (C-4) 82.1 (C-5), 135.6 (C-6), 130.9 (C-7), 79.1 (C-8), 50.8 (C-9), 37.1 (C-10). 23.1 (C-11), 40.5 (C-12), 45.8 (C-13), 52.1 (C-14), 21.9 (C-15), 29.7 (C-16), 55.9 (C-17), 12.8(C-18), 18.3 (C-19), 39.9(C-20), 20.9 (C-21), 137.8(C-22), 126.9 (C-23), 40.9 (C-24), 29.1 (C-25), 22.8 (C-26), 21.9 (C-27), 21.2 (C-28).
3.4. Antibacterial Activity
The crude extract and pure compounds were separately dissolved in MeOH to obtain 1000-mg/mL sample stock solutions. Four human bacterial pathogens were used for antibacterial activity, including B. subtilis (ATCC 6633), P. aeruginosa (ATCC 27853), S. aureus (ATCC 25923), and E. coli (ATCC 25922).
The Disc Diffusion Assay and the Minimum Inhibition Concentration (MIC) tests were performed as described by Putra and Hadi (13), with slight modifications. For the Disk Diffusion Assay, 20 µL of the sample was added onto a paper disc, then placed on Mueller Hinton agar (Himedia) in a petri dish inoculated with the test bacterial pathogen. The inhibition zone was measured using calipers after 24 hours of incubation at 37°C. All the assays were conducted in triplicate.
The MIC test was performed using serial dilutions from 5, 10, 100, 250, 500 μg/mL of the test compound in sterile 96-well plates filled with Mueller-Hinton broth medium (MHB). As a control, we used ampicillin (10 μg/mL) and methanol. After 24 h of incubation, each well's turbidity was measured using a microplate reader at 600 nm.
3.5. Cytotoxic Activity
3.5.1. HCT 116 Cytotoxic Assay
The colon cancer adenocarcinoma HCT 116 cell line was cultured in DMEM medium with 10% FBS and put in an incubator at 37°C with 5% CO2. HCT-116 cells (1 x 104 cells/ well) were seeded in 96-well plates. The samples in 0.5-L aliquots diluted in DMSO were added to wells at varying concentrations after 24 h. For cytotoxicity controls, doxorubicin and DMSO were used. After 48 h of incubation, the plates were developed with MTT dye based on the manufacturer's protocol (14).
3.5.2. Brine Shrimp Lethality Assay (BSLA)
An in vitro brine shrimp cytotoxic assay was performed to examine the toxicity of the crude extracts and fractions (15). Briefly, Artemia salina (brine shrimp) was hatched in 500 mL of seawater at room temperature. The concentration samples were prepared at the concentrations of 1, 10, and 100 µg/mL for the crude extract and the concentrations of 5, 25. 50, 100, and µg/mL for the pure compounds. A total of 10 larvae were incubated for 24 h in each vial with 5 mL. Finally, the IC50 was calculated for cytotoxic activity.
4. Results and Discussion
From the bioassay-guided isolation of the bioactive compounds from Sinularia polydactila by antibacterial and cytotoxic activity assay, nine fractions from Si-gel column chromatography exhibited low to significant inhibitory activity on human colon cancer HCT 116 cells, brine shrimp lethality assay, and four human pathogenic bacteria. The fraction obtained using n-Hexane: EtOAc (40:60) was selected for the isolation of the main compounds owing to showing the highest antimicrobial and cytotoxic activities. Compounds 1 and 2 were isolated from the active fraction. Structure elucidation of those compounds was performed by NMR (1D and 2D Data) and mass spectrometry. All the spectroscopic data from the isolated compounds were compared with reference data in the literature.
4.1. Structure Elucidation
Compound 1 was achieved as a white amorphous powder and identified as (22R,23R,24R)-22,23-methylene-24-methylcholest-6-en-5α,8α-epidioxy-3β-ol with at m/z 465.34 [M+Na]+, suggesting C29H46O3 as the molecular formula (seven degrees of unsaturation). This compound possesses 29 carbons, including six methyls, eight sp3 methylenes, nine sp3 methines, two sp2 methines, and four sp3 quaternary carbons. Two singlet methyl groups, δH 0.76 (3H, s, H-18) and δH 0.90 (3H, s, H-19), three doublet methyls at δH 0.91 (6H, d, J = 6.3 Hz, H-21), 0.85 (6H, d, J = 6.3 Hz, H-26), 0.89 (3H, d, J = 6.9 Hz, H-27), 0.91 (3H, d, J = 6.9 Hz, H-28), were attributed to Me-21, Me-26, Me-27 and Me-28, respectively. The characteristic signals 6.24 (1H, d, J = 8.4 Hz, H-6), 6.51 (1H, d, J = 8.4 Hz, H-7) and an oxygenated methine at δH 3.98 (1H, m, H-3) in proton NMR suggested this compound as a 3β-hydroxy-6-en-5α,8α-epidioxysterol nucleus. Since the signals for protons H-22/H-24, H-20/H3-21, and H3-26, H3-27 overlapped in the 1H spectrum of 1, it is difficult to use the NOESY spectrum by their NOE effect to determine the relative configuration of the cyclopropyl moiety. Nevertheless, by comparison of the 1H- and 13C-NMR chemical shifts of Me-21, Me-26, Me-27, and Me-28 with those of a known epidioxysterol, compound 1 was identified as (22R,23R,24R)-5α,8α-epidioxy-22,23-methylene-24-methylcholest-6-en-3β-ol, which has been previously identified from a Formosan soft coral Sinularia spp. (16).
Compound 2 was identified as (22E,24S)-5,8- epidioxy-24-methyl-cholesta-6,22-dien-3-ol. The ESIMS showed peak m/z 451.54 [M+Na]+, suggesting the molecular formula C28H44O3 (seven degrees unsaturation). Compound 2 was identified as peroxysteroid, because in the 13C-NMR showed three oxygenated C atoms at δC 82.7 (C-5), 78.4 (C-8) and 66.3 (CH-3) in the carbon NMR spectrum and an oxymethine proton at δH 3.97 (1H, m, H-3) in the proton NMR spectrum. Compound 2 possesses an endocyclic double bond between the carbons C-6 and C-7, and a peroxy group between C-5 and C-8. This sterol was further recognized as a peroxysteroid containing a 22,23-methylene-24-methyl moiety in the side chain by the presence of characteristic signal for H-22 and H-23 at δH and 5.22 (1H, d, J = 15.1 Hz, H-22), 5.13 (1H, d, J = 15.1 Hz, H-23), and four doublets at δ 0.91 (3H, d, J = 7.2 Hz, H-28), 0.81 (3H, d, J = 6.6 Hz, H27), 1,01 (3H, d, J = 6.4 Hz, H-21), and 0.82 (3H, d, J = 6.6 Hz, H-26), respectively. In comparison with the spectroscopic data from the described literature, compound 2 was identified as (22E,24S)-5,8- epidioxy-24-methyl-cholesta-6,22-dien-3-ol (17).
4.2. Antimicrobial Activity
Samples of Sinularia polydactila (500 g wet weight) were extracted with MeOH and dichloromethane. Nine fractions were yielded from gravity chromatography using silica gel as a stationary phase and mobile phase from n-hexane to EtOAc to MeOH. The fractions obtained were tested for antimicrobial and cytotoxic activities by the bioassay-guided isolation of bioactive compounds.
The results indicated that fractions obtained with n-Hexane: EtOAc (40:60) showed potent antibacterial activity against B. subtilis and S. aureus (Table 1). The fractions were further purified by HPLC to obtain two polyhydroxylated steroids, compounds 1and 2, as identified above. They were examined for antibacterial activity by determining the minimum inhibitory concentration (MIC). 24-methylene-cholesterol (3) showed moderate activity against S. aureus and B. subtilis at a concentration of 250 μg/mL (Table 2). Wei et al. (18) identified the same compound that displayed antitubercular activity with IC50 of 120.1 μg/mL.
Antibacterial Activity as Determined by the Disk Diffusion Assay
| No. | Fractions/Compounds | Zone of Inhibition (mm) | |||
|---|---|---|---|---|---|
| S. aureus | B. subtilis | E. coli | P. aeruginosa | ||
| 1 | n-hexane | 7.65 ± 1.63 | 10.20 ± 0.85 | 6.38 ± 0.46 | 9.53 ± 0.18 |
| 2 | n-hexane:ethyl acetate (8:2) | 7.23 ± 0.29 | 8.46 ± 0.22 | 10.25 ± 0.13 | 7.81 ± 0.23 |
| 3 | n-hexane:ethyl acetate (6:4) | 8.56 ± 0.37 | 10.35 ± 0.36 | 10.23 ± 0.49 | 10.11 ± 0.20 |
| 4 | n-hexane:ethyl acetate (4:6) | 14.53 ± 0.51 | 14.48 ± 0.48 | 11.98 ± 0.74 | 11.78 ± 0.33 |
| 5 | Ethyl acetate: n-hexane (2:8) | 9.75 ± 0.91 | 10.93 ± 0.71 | 9.21 ± 0.18 | 9.17 ± 0.18 |
| 6 | Ethyl acetate | 9.78 ± 0.39 | 8.33 ± 1.59 | 9.50 ± 0.21 | 7.18 ± 1.24 |
| 7 | Ethyl acetate:methanol (8:2) | 11.77 ± 1.65 | 10.61 ± 0.86 | 7.45± 0.54 | 6.79 ± 0.86 |
| 8 | Ethyl acetate:methanol (6:4) | 8.34 ± 1.37 | 11.48 ± 1.31 | 6.17 ± 0.25 | 9.10 ± 0.11 |
| 9 | Ethyl acetate:methanol (2:8) | 7.48 ± 0.41 | 6.48 ± 1.54 | 7.93 ± 2.21 | 9.34 ± 0.09 |
| 10 | (22R,23R,24R)-22,23-methylene-24-methylcholest-6-en-5a,8a-epidioxy-3b-ol (1) | 6.24 ± 1.41 | 07.48 ± 2.01 | 6.68 ± 0.35 | 9.08 ± 0.12 |
| 11 | (22E,24S)-5,8- epidioxy-24-methyl-cholesta-6,22-dien-3-ol (2) | 7.67 ± 0.52 | 5.58 ± 1.63 | 5.43 ± 1.91 | 8.14 ± 0.19 |
| 12 | Ampicillin | 14.48 ± 1.31 | 30.11 ± 1.31 | 20.93 ± 1.21 | 19.24 ± 0.16 |
Cytotoxic Data of Sterols
| Compounds | BSLA, LC50 (μg/mL) | HCT 116, IC50 (μg/mL) |
|---|---|---|
| 1 | 57.1 | 24.8 |
| 2 | 121.3 | 27.3 |
| Doxorubicin | - | 0.22 |
4.3. Cytotoxic activity
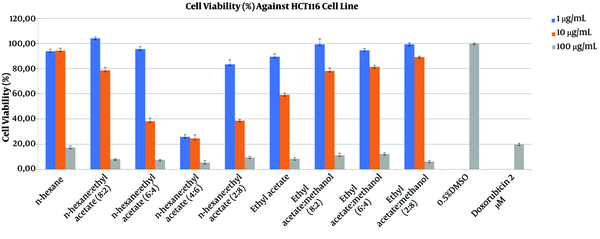
Cytotoxicity assays were performed for nine fractions as shown in Table 1 at the concentrations of 1, 10, and 100 μg/mL (Figure 2). The viability of HCT 116 cells decreased by more than 60% after 48 h of exposure to the EtOAc fraction (10 μg/mL). However, as in the case with the antibacterial assay, the most active fraction for cytotoxicity was n-Hexane:EtOAc (4:6).
% Cell Viability of HCT 116
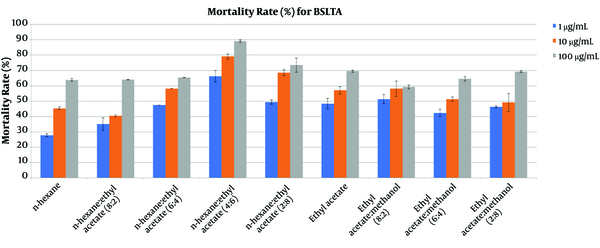
The brine shrimp lethality results for the fractions of the crude extract from soft coral Sinularia polydactila are presented in Figure 3. The most active fraction that showed cytotoxic properties was n-Hexane: EtOAc (4:6), showing a more than 70% mortality rate at a concentration of 10 μg/mL. The results indicated a positive correlation between cytotoxic and antibacterial activities in the same fraction.
% Mortality rate for BSLA
The purified compounds were further analyzed for cell proliferation inhibitory effect. Compounds 1 and 2 showed moderate activity against colon carcinoma cancer HCT 116, with IC50 at the concentrations of 24.8 and 27.3 μg/mL, respectively (Table 2). The brine shrimp lethality results for the compounds 1 and 2 showed cytotoxic properties, with the LC50 values of 57 and 121.3 μg/mL, respectively (Table 2).
A previous study explained Compound 1 in the soft coral Sinularia spp., collected from Taiwan. This compound had significantly inhibited HT 29, KB, P-388, and A549 at the concentrations of 1.4, 2.1, 0.4, and 2.7 μg/mL, respectively (19).
5. Conclusions
The chemical study from the organic extracts of Indonesian Sinularia polydactila resulted in two known compounds (22R,23R,24R)-22,23-methylene-24-methylcholest-6-en-5a,8a-epidioxy-3b-ol (1) and 5a,8a-Epidioxy-24(R)-methylcholesta-6,22-dien-3a-ol (2). These two metabolites showed moderate cytotoxic activities against HCT 116 and the brine shrimp lethality assay (BSLTA) from the biological activities. The cytotoxic activities for compounds 1 and 2 using the brine shrimp lethality resulted in the LC50 values of 57 and 121.3 μg/mL, respectively. Furthermore, Compounds 1 and 2 showed the IC50 values of 24.8 and 27.3 μg/mL, respectively, against colon carcinoma cancer HCT 116. Owing to these attractive biological activities, compounds 1 and 2 might be useful in the future to study and explain the cytotoxic properties of sterol or steroidal compounds for drug discoveries.
References
-
1.
Mehdinia A, Sheijooni Fumani N, Rezaei H. [Essential oils of a soft coral (Sinularia sp) from Chabahar Bay of Iran]. J Persian Gulf. 2014;5(15):51-8. Persian.
-
2.
Blunt JW, Copp BR, Keyzers RA, Munro MH, Prinsep MR. Marine natural products. Nat Prod Rep. 2013;30(2):237-323. [PubMed ID: 23263727]. https://doi.org/10.1039/c2np20112g.
-
3.
Chen W, Li Y, Guo Y. Terpenoids of Sinularia soft corals: chemistry and bioactivity. Acta Pharmaceutica Sinica B. 2012;2(3):227-37. https://doi.org/10.1016/j.apsb.2012.04.004.
-
4.
Huang CY, Su JH, Duh CY, Chen BW, Wen ZH, Kuo YH, et al. A new 9,11-secosterol from the soft coral Sinularia granosa. Bioorg Med Chem Lett. 2012;22(13):4373-6. [PubMed ID: 22672798]. https://doi.org/10.1016/j.bmcl.2012.05.002.
-
5.
Su J, Lo C, Lu Y, Wen Z, Huang C, Dai C, et al. Anti-inflammatory polyoxygenated steroids from the soft coral Sinularia sp. Bull. Chem. Soc. Jpn. 2008;81(12):1616-20. https://doi.org/10.1246/bcsj.81.1616.
-
6.
Cheng S, Chen H, Wang S, Duh C. Three new 9,11-secosterols from the formosan soft coral Sinularia leptoclados. Bull Chem Soc Jpn. 2011;84(6):648-52. https://doi.org/10.1246/bcsj.20110046.
-
7.
Ngoc NT, Huong PT, Thanh NV, Chi NT, Dang NH, Cuong NX, et al. Cytotoxic steroids from the vietnamese soft coral Sinularia conferta. Chem Pharm Bull (Tokyo). 2017;65(3):300-5. [PubMed ID: 28077809]. https://doi.org/10.1248/cpb.c16-00881.
-
8.
Tran HHT, Nguyen Viet P, Nguyen Van T, Tran HT, Nguyen Xuan C, Nguyen Hoai N, et al. Cytotoxic steroid derivatives from the Vietnamese soft coral Sinularia brassica. J Asian Nat Prod Res. 2017;19(12):1183-90. [PubMed ID: 28421816]. https://doi.org/10.1080/10286020.2017.1307192.
-
9.
Rajaram S, Ramesh D, Ramulu U, Anjum M, Kumar P, Murthy USN, et al. Chemical examination of the soft coral Sinularia kavarattiensis and evaluation of anti-microbial activity. Indian J Chem Sect B. 2014;53(8):1086-90.
-
10.
Izzati F, Warsito MF, Bayu A, Prasetyoputri A, Atikana A, Sukmarini L, et al. Chemical diversity and biological activity of secondary metabolites isolated from Indonesian marine invertebrates. Molecules. 2021;26(7). [PubMed ID: 33801617]. [PubMed Central ID: PMC8037762]. https://doi.org/10.3390/molecules26071898.
-
11.
Fernandez R, Bayu A, Aryono Hadi T, Bueno S, Perez M, Cuevas C, et al. Unique polyhalogenated peptides from the marine sponge Ircinia sp. Mar Drugs. 2020;18(8). [PubMed ID: 32731567]. [PubMed Central ID: PMC7460063]. https://doi.org/10.3390/md18080396.
-
12.
Pollastro F, Golin S, Chianese G, Putra MY, Schiano Moriello A, De Petrocellis L, et al. Neuroactive and anti-inflammatory frankincense cembranes: A structure-activity study. J Nat Prod. 2016;79(7):1762-8. [PubMed ID: 27352042]. https://doi.org/10.1021/acs.jnatprod.6b00141.
-
13.
Putra MY, Hadi TA. Chemical composition, antimicrobial, cytotoxic and antiplasmodial activities of three sponges from Buton Islands, Indonesia. ILMU KELAUTAN. 2017;22(3). https://doi.org/10.14710/ik.ijms.22.3.147-154.
-
14.
Liu Y, Salvador LA, Byeon S, Ying Y, Kwan JC, Law BK, et al. Anticolon cancer activity of largazole, a marine-derived tunable histone deacetylase inhibitor. J Pharmacol Exp Ther. 2010;335(2):351-61. [PubMed ID: 20739454]. [PubMed Central ID: PMC2967399]. https://doi.org/10.1124/jpet.110.172387.
-
15.
Mahmud S, Paul GK, Afroze M, Islam S, Gupt SBR, Razu MH, et al. Efficacy of phytochemicals derived from Avicennia officinalis for the management of COVID-19: A combined in silico and biochemical study. Molecules. 2021;26(8). [PubMed ID: 33921289]. [PubMed Central ID: PMC8070553]. https://doi.org/10.3390/molecules26082210.
-
16.
Sheu JH, Chang KC, Duh CY. A cytotoxic 5alpha,8alpha-epidioxysterol from a soft coral Sinularia species. J Nat Prod. 2000;63(1):149-51. [PubMed ID: 10650100]. https://doi.org/10.1021/np9903954.
-
17.
Gauvin A, Smadja J, Aknin M, Faure R, Gaydou E. Isolation of bioactive 5α,8α-epidioxy sterols from the marine sponge Luffariella cf. variabilis. Can J Chem. 2000;78(7):986-92. https://doi.org/10.1139/v00-083.
-
18.
Wei X, Rodriguez AD, Wang Y, Franzblau SG. Synthesis and in vitro biological evaluation of ring B abeo-sterols as novel inhibitors of Mycobacterium tuberculosis. Bioorg Med Chem Lett. 2008;18(20):5448-50. [PubMed ID: 18818073]. [PubMed Central ID: PMC2634294]. https://doi.org/10.1016/j.bmcl.2008.09.029.
-
19.
Yen WH, Chen WF, Cheng CH, Dai CF, Lu MC, Su JH, et al. A new 5alpha,8alpha-Epidioxysterol from the soft coral Sinularia gaweli. Molecules. 2013;18(3):2895-903. [PubMed ID: 23459300]. [PubMed Central ID: PMC6270315]. https://doi.org/10.3390/molecules18032895.