1. Background
The liver is a vital and the biggest internal organ of the body and has several functions, including being a source for the production of blood components, detoxifying blood-born bacteria and toxins, facilitating drug and food metabolism, synthesis of lipids, glucose, proteins, and vitamins, iron supply, synthesizing coagulation factors, and bile production. Therefore, liver dysfunction may result in damage to other tissues in the body (1).
Exposure to environmental toxicants, certain medicinal agents, alcohol, and microbial metabolites has been reported to cause liver damage. Liver injury is associated with systemic oxidative stress, leading to cellular necrosis, fibrosis, lipid peroxidation, and cellular glutathione depletion (2-4).
Acetaminophen (APAP)-induced toxicity is one of the most important causes of acute liver failure (ALF) (5). Acetaminophen, as an antipyretic and analgesic medicine, is commonly used worldwide and is safe in therapeutic doses (up to 4 g); however, its overdose can lead to serious liver injury (6). Via the action of some enzymes such as CYP450, APAP metabolization leads to the production of a toxic compound called N-acetyl-P-benzo quinone imine (NAPQI) that is subsequently detoxified by being conjugated with glutathione. Increased levels of NAPQI because of the saturation of conjugation pathways lead to glutathione (GSH) depletion, resulting in protein damage, oxidative stress, mitochondrial damage, and Kuepfer cells’ activation, finally culminating in liver damage (7, 8). Hepatocytes can regenerate and reproduce themselves, but in overdoses, due to the acute damage and slow regenerative processes, they are not able to compensate for injuries. Liver protectors, such as antioxidants, help neutralize free radical species to save hepatocytes (9, 10). Therefore, antioxidant compounds are among the most important agents protecting tissues, including the liver, against oxidative damage. Currently, N-acetyl cysteine (NAC) is used as an antidote to APAP, which is a substrate for GSH production, helping to detoxify NAPQI. From the onset of APAP poisoning, the effects of NAC start to fade. Plant antioxidant compounds can usually be replaced via several effective auxiliary pathways and mechanisms. Crocin, a water-soluble carotenoid pigment (red-colored saffron), is one of these plant compounds and has different biological effects (11, 12). For example, it can act as an antioxidant by inhibiting the activity of free radicals and xanthine oxidase. Crocin is also used as a supplementary agent to treat inflammatory diseases due to its anti-inflammatory effects (13). Crocin has also been reported to have anti-inflammation (14), anti-arthritis (15), anti-cancer (16), anti-atherosclerosis (17), and hypolipidemic (18) effects and enhance neuronal and memory function (19). According to these effects, this research aimed to evaluate the protective effects of crocin against APAP-induced oxidative stress in mice.
2. Methods
2.1. Animals
In this study, 56 male Swiss albino mice weighing 25 ± 3 grams were used. The mice were obtained from the animal house of Jundishapur University of Medical Sciences, Ahvaz, Iran, and kept in cages under controlled temperature (20 ± 2°C) and a 12 h light: 12 h dark cycle. This study was performed according to the guidelines of the Animal Ethics Committee of Ahvaz Jundishapur University of Medical Sciences (ethical approval number: IR.AJUMS.REC.1396.677).
2.2. Materials
Purified crocin powder was purchased from Sigma Company (USA) and diluted in normal saline to prepare the desired doses. Acetaminophen powder was purchased from Dr. Abidi Pharmaceutical Company (Iran) and dissolved in warm normal saline.
2.3. Experimental Design
The mice were randomly classified into seven groups (N = 8 per group). The first (control) group received normal saline (10 mg/kg, 2 mL) for six days; the second group (APAP) received normal saline for five days + acetaminophen (300 mg/kg) on day 6th, and the third group (NAC) was treated with normal saline for five days and then on day 6th received NAC (50 mg/kg) half an hour before and immediately after the administration of acetaminophen. The groups four to six received 12.5, 25, and 50 mg/kg crocin, respectively, for six days, followed by APAP (300 mg/kg) on day 6th, 30 minutes after the last crocin administration. The last group received crocin (50 mg/kg) for six days. All compounds were injected intraperitoneally (20, 21).
2.4. Measurement of Biochemical Indicators
To determine the activity of ALT and AST enzymes, sera, after freezing, were sent to a private laboratory. The tissues were homogenized before measuring histological parameters. First, tissues were weighed, then mixed with a phosphate buffer with the ratio of 1 to 10 (0.1 M and pH = 7.4), and homogenized by a homogenizer at 740rpm for 90 seconds. Then the obtained mixture was centrifuged at 4000 rpm for 10 minutes, and each sample was divided into four microtubes to measure tissue indicators (22).
2.5. Oxidative Stress Markers
2.5.1. Glutathione
Glutathione measurement was performed using the Ellman method. First, 1 mL of homogenized samples was mixed with 1 mL of TCA 20% (trichloroacetic acid) and 1 mL of EDTA, and after five minutes, the mixture was centrifuged at 3000 rpm for 15 minutes. Then 200 μL of the supernatant was mixed with 1.8 μL DTNB (5,5'-dithiobis-(2-nitrobenzoic acid) 0.1 M, and absorbance was read at 412 nm by a spectrophotometer (23).
2.5.2. Lipid Peroxidation Assay
The Satho method was used to determine malondialdehyde level. For this, 0.5 mL of homogenized samples was added to 1.5 mL of TCA 10%, and then the mixture was centrifuged at 4000 rpm for 10 minutes. To 1.5 mL of the supernatant, 2 mL of TBA 76% (thiobarbituric acid) was added, and then it was incubated in boiling water for 30 minutes. At 532 nm, a spectrophotometer was used to read the absorbance of the pink complex (24, 25).
2.5.3. Antioxidant Enzymes
2.5.3.1. CAT
This test was performed by the Aebi procedure. In a cuvette containing 200 μL phosphate buffer and 50 μL tissue supernatant, 250μL of 0.066 M H2O2 was added (as the substrate), and a decrease in optical density (OD) was measured spectrophotometrically at 240 nm for one minute. Finally, CAT activity was presented as µM H2O2 consumed/min/mg protein (26, 27).
2.5.3.2. GPX
The Randox commercial kit (the UK) was used to measure glutathione peroxidase activity.
2.5.3.3. Protein Content
The protein content of samples was measured by the Bradford method (22).
2.6. Histological Examination
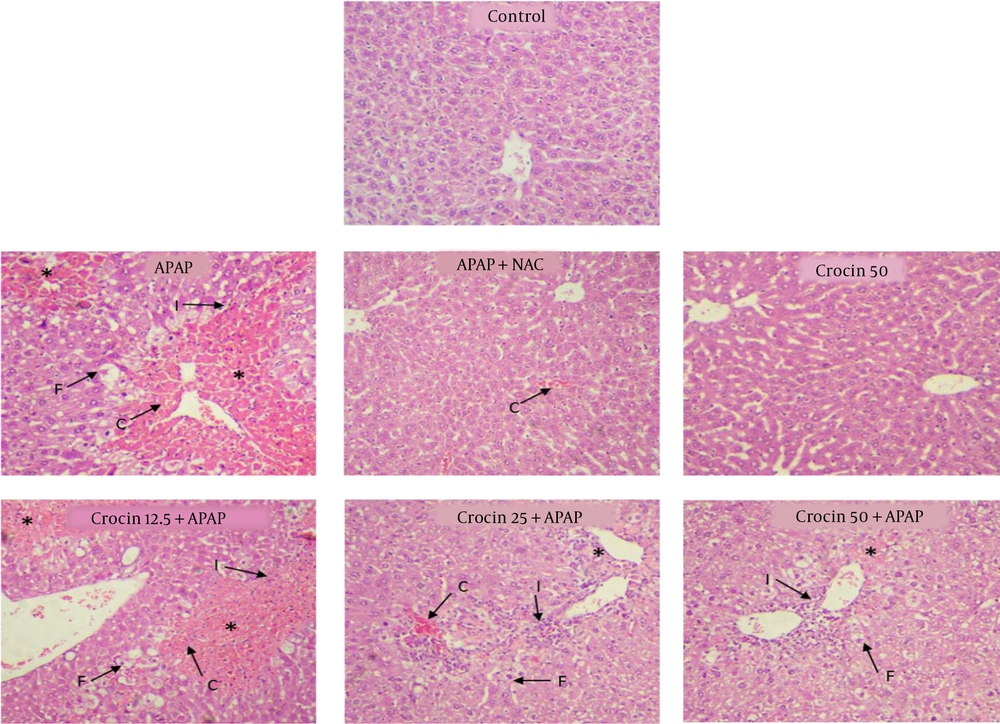
For histological examination, lung samples were removed immediately after sacrifice, fixed in a 10% formalin solution, dehydrated in graded alcohol concentrations, and embedded in paraffin. Sections with 4 - 6 µm thickness were prepared and stained with hematoxylin and eosin (H&E). Six microscopy slides per animal were examined for assessing histological changes such as erythrocyte congestion, infiltration by inflammatory cells, fat deposition in hepatocytes, and necrosis. Histological features were graded into four categories: (1) normal (0), (2) weak (1), (3) moderate (2), or (4) intense (3), based on the average scores obtained by examining six microscopic fields by a researcher who was blind to the group of the sample.
2.7. Statistical Analysis
Evaluated parameters were presented by mean ± SEM, and the ANOVA test was used to compare them. To determine a significant difference between the groups, Tukey’s post-hoc test was used, and a P value of < 0.05 was regarded as the statistical significance level.
3. Results
3.1. Biochemical Findings
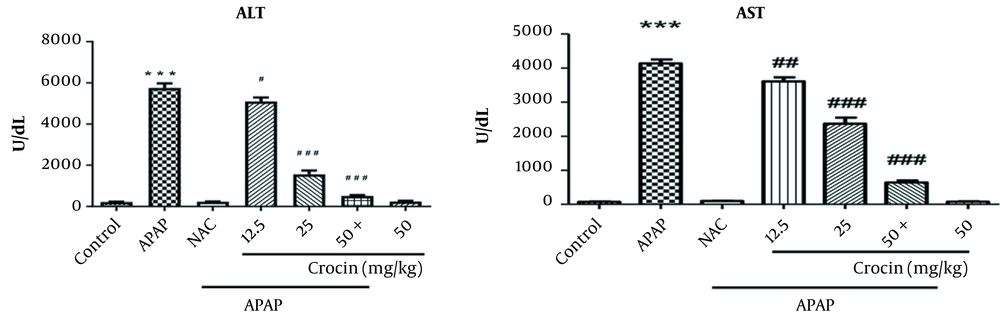
Regarding the mean value of ALT, APAP caused a significant increase in this serum marker compared to the control group (P < 0.001). However, there was a significant reduction in serum ALT in APAP + crocin receiving groups (at all three doses) compared to the APAP-treated group (P < 0.05). The group receiving crocin alone did not show a significant change in ALT compared to the control group (Figure 1).
The mean level of serum AST showed a statistically significant increase in the APAP group compared to the control group (P < 0.001) but significantly decreased in the groups treated with APAP + crocin (at all three doses) compared to the APAP-exposed group (P < 0.01). The last experimental group (i.e., crocin alone) did not show a significant change in serum AST level (Figure 1).
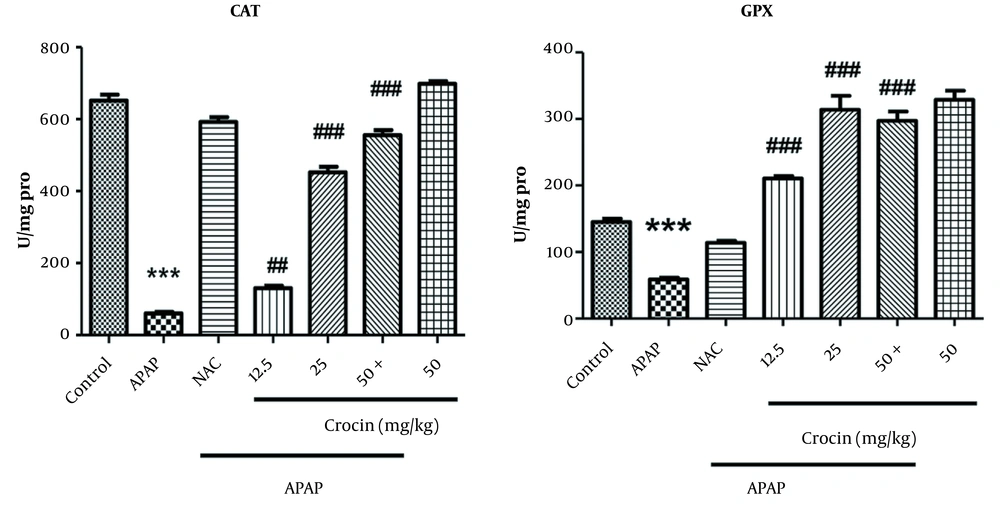
Mean GPX enzyme activity was measured in different groups, which showed that APAP treatment significantly reduced the activity of this enzyme compared to the control group (P < 0.001). On the other hand, a significant increase in GPX activity was observed in the mice treated with crocin (i.e., groups four to six) compared to the animals exposed to APAP (P < 0.001), with significantly higher activities at the doses of 25 and 50 mg/kg compared to the dose of 12.5 mg/kg (P < 0.001, Figure 2).
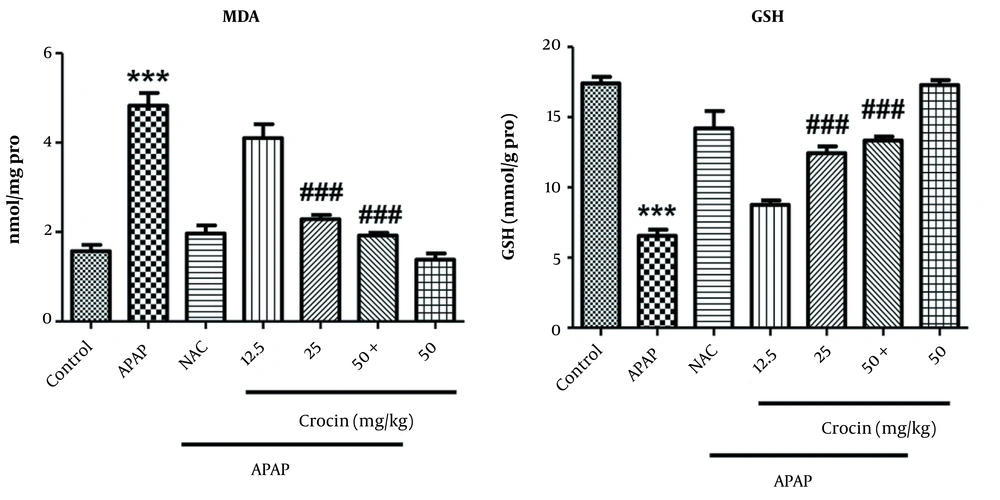
Mean MDA level also elevated significantly in the APAP group compared to the control group but showed a meaningful reduction in the groups treated with the doses of 25 and 50 mg/kg compared to the APAP group (P < 0.001). The crocin group did not show a significant change (Figure 3). The measurement of GSH level in different groups showed that APAP significantly reduced the level of this indicator compared to the control group (P < 0.001). In the groups receiving crocin 25 and 50 mg/kg, GSH level increased compared to the APAP group (P < 0.001). The efficacy of these two doses in boosting GSH was comparable with NAC, and the latter group did not show a significant difference compared with the control group (Figure 3).
In addition, APAP treatment reduced CAT level (P < 0.001) while all three doses of crocin significantly increased the level of this enzyme compared to the APAP group (P < 0.01), but the efficacy of 25 and 50 mg/kg doses was significantly higher than the dose of 12.5 mg/kg (i.e., a dose-dependent mode of action). In the group receiving crocin alone, this compound did not exert any negative effect on CAT level (Figure 2).
3.2. Histopathologic Findings
In histopathologic studies, the cells were completely normal and healthy in the control and crocin (50 mg/kg)-treated groups. However, in the group receiving APAP, necrosis, fat deposition, infiltration by inflammatory cells, and erythrocyte congestion were observed (P < 0.001). In the NAC + APAP group, all of the above-mentioned parameters were observed but with lesser severity (P < 0.01). In the groups receiving APAP and crocin (25 and 50 mg/kg), the severity of the lesions decreased significantly compared to the APAP group (P < 0.05). In the group receiving 12.5 mg/kg crocin, histopathological lesions were similar to the APAP group, and no significant improvement was observed (Table 1, Figure 4).
| Groups | Histological Criteria | |||
|---|---|---|---|---|
| Erythrocyte Congestion | Inflammation | Fat Deposition | Necrosis | |
| Control | 0.02 ± 0.00 | 0.03 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| APAP | 2.32 ± 0.36*** | 2.1 ± 0.31*** | 2.43 ± 0.42*** | 2.73 ± 0.52*** |
| APAP + NAC | 0.22 ± 0.04** | 0.11 ± 0.02** | 0.03 ± 0.00** | 0.04 ± 0.00** |
| Crocin 50 | 0.01 ± 0.00 | 0.02 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Crocin 12.5 + APAP | 2.12 ± 0.36*** | 1.93 ± 0.25*** | 2.41 ± 0.44** | 2.12 ± 0.52*** |
| Crocin 25 + APAP | 0.92 ± 0.26***## | 1.03 ± 0.15*# | 0.61 ± 0.14**## | 0.81 ± 0.22***## |
| Crocin 50 + APAP | 0.22 ± 0.26**### | 0.43 ± 0.15***## | 0.33 ± 0.14***### | 0.28 ± 0.22**### |
a *Significant difference in comparison with the control group (***P < 0.001).
b Significant difference in comparison with the APAP group (#P < 0.05, ##P < 0.01, ###P < 0.001).
4. Discussion
Acetaminophen is an metabolized mainly by Cyt P450 enzymes to a toxic metabolite called N-acetyl-p-benzoquinoneimine (NAPQI) that can be detoxified by being conjugated with reduced GSH. However, acute overdose or GSH insufficiency allows NAPQI to bind hepatic proteins, leading to oxidative stress, mitochondrial dysfunction, and hepatic necrosis (28). Previous studies have shown that APAP leads to an oxidant-antioxidant imbalance in the liver, during which increased oxidative stress results in tissue damage such as cellular necrosis, biochemical changes like glutathione depletion, an increase in the production of proxy nitrite and active oxygen species, increased inflammatory factors, and reduced ATP production (29-31). Due to the destructive effects of active oxygen and nitrogen species, modifying their effects by antioxidants, such as crocin, can be a helpful therapeutic strategy. Therefore, compounds with antioxidant and anti-inflammatory properties have acquired great attention. Saffron is a medicinal plant used for a long time to alleviate ailments due to its extensive therapeutic effects (32-34). Crocin is the main and most important therapeutic ingredient of saffron, which has many properties, including antioxidant effects (35-37). In this study, we studied the protective effects of crocin on APAP-induced liver damage by examining biochemical and histopathological parameters.
Our results showed that APAP administration (300 mg/kg) increased serum levels of AST, ALT, and MDA, decreased GSH content and caused necrosis, fat deposition, infiltration by inflammatory cells, and erythrocyte congestion, which all reversed by crocin treatment.
Oxidative stress has been accepted as an important molecular mechanism of APAP-induced hepatotoxicity by promoting cell membrane damage and the consequent release of enzymatic markers of hepatotoxicity, mitochondrial dysfunction, and lipid accumulation in hepatic cells, leading to inflammation and subsequently steatosis and steatohepatitis (38).
Glutathione, a non-enzymatic thiol antioxidant, serves as an important line of defense against the oxidative stress caused by xenobiotics. It donates electrons to hydrogen peroxide (H2O2) to reduce it to H2O, which finally results in its detoxification, inhibiting lipid peroxides and protecting cell membranes against lipid peroxidation (39). As the most important oxidant by-product of peroxidized polyunsaturated fatty acids, MDA is routinely used to evaluate the presence of free radicals and lipid peroxidation-induced toxicity. It seems that the administration of APAP leads to the generation of lipid peroxidation products like MDA, which interact with membrane lipids and consequently induce free radicals’ formation, leading to oxidative stress in the liver (as the main site for APAP metabolic biotransformation) (40). Our results revealed that APAP treatment decreased the level of GSH (an antioxidant marker) and increased MDA level (a lipid peroxidation marker); however, crocin administration markedly prevented these changes. The body’s cells have an effective defense mechanism against ROS (e.g., hydroxyl radical (OH•), superoxide anion (O2•−), hydrogen peroxide (H2O2), and singlet oxygen), which contains a set of antioxidant enzymes such as SOD and CAT that play key roles in maintaining the oxidative–antioxidative balance (41). A significant decrease in the activity of SOD, CAT, and GPx may lead to the direct conjugation of APAP to free or protein-bound –SH groups (42, 43). Crocin restored the levels of non-enzymatic (GSH) and enzymatic antioxidants (SOD, CAT, and GPx), indicating that its hepatoprotective effects against APAP-induced liver injury may be partly due to its antioxidant properties and ability to reduce and scavenge the free radicals produced in the liver (38).
Aminotransferases (ALT, AST) are serum hepatic biomarkers that are significantly altered during liver damage (29). Elevated AST and ALT indicate damage to the hepatocellular plasma membrane and are considered good markers for liver injury, inflammation, and necrosis. Our findings showed that crocin administration prevented APAP-induced liver histopathological changes as evidenced by H&E staining and reduction in AST and AST levels.
Many studies support the protective role of crocin against oxidative stress-induced deleterious cellular consequences. The results of a study by Lari et al. in 2015 showed that 20 mg/kg crocin reduced the toxic effects of diazocin on the liver of rats (11). Another study by Salahshoor et al. in 2016 found that crocin (25.5, 25, 50 mg/kg) alleviated morphine-induced liver damage in mice (21). Also, Afolabi et al. in 2016 showed that crocin at the doses of 20 and 40 mg/kg decreased 5-FU-induced liver damage in rats (44).
It seems that crocin treatment can reverse elevated liver enzymes and the antioxidant balance to near-normal by decreasing lipid peroxidation, scavenging ROS, and enhancing cellular antioxidant enzymes and GSH content. Furthermore, crocin protective effects may be due to its role in parenchymal cell regeneration in the liver, protecting membrane integrity and thereby decreasing enzyme leakage. So, the hepatoprotective activity of crocin can root in a variety of actions, including the modulation of ROS production and the antioxidant status and blocking the metabolic activation of carcinogens (45).
4.1. Conclusions
We here showed that, in line with previous studies, APAP caused damage to the liver tissue through boosting oxidative stress. Our biochemical and pathological findings indicated that crocin had dose-dependent protective effects against APAP-induced liver toxicity in Swiss albino male mice, mainly through antioxidant effects. It seems that this substance can be used as a complementary drug with NAC; further studies are needed to confirm our results.