1. Background
Polycystic ovary syndrome (PCOS) is one of the most common endocrine disorders that affects 1 in 10 women of reproductive age, and the etiology of this syndrome is unknown (1). The characteristics of the syndrome include menstrual abnormalities, high androgen levels, hirsutism, obesity, and polycystic ovarian morphology (2). Infertility in women with PCOS due to anovulation is one of the main complications caused by this disease. However, PCOS complications extend beyond fertility concerns. The patients are also at increased risk for type 2 diabetes, hypertension, cardiovascular disease, and endometrial cancer (3-5).
PCOS treatment includes various aspects of the disease, such as reproductive, metabolic, and psychological features. For instance, lifestyle modifications (diets and exercise) to treat metabolic disorders (6) and pharmacologic therapies, including metformin, oral contraceptives (OCPs), and anti-androgen drugs (such as spironolactone and cyproterone acetate) (7), are used to control the disease. However, because of the adverse effects of these drugs, such as lactic acidosis (8), weight gain, cardiovascular disease, thromboembolism, and the relative low responses to the current therapies (9, 10), it is crucial to consider alternative treatments with fewer side effects and more effectiveness (11).
Today, due to the remarkable effects of phytopharmaceuticals in managing diseases, the use of these compounds is increasing (12). In Iranian traditional medicine (ITM), many special herbal medicines have been used to prevent and cure PCOS (11, 13). Thus far, many scientific studies have proved the effect of Iranian herbal drugs in PCOS, such as Apium graveolens L. (14, 15), Pimpinella anisum L. (15, 16), Vitex agnus-castus L. (17), Trigonella foenum-graecum L. (18), Cinnamomum verum J. Presl (19), and Foeniculum vulgare Mill. (20, 21).
Fennel (F. vulgare Mill.), anise (P. anisum L.), and celery (A. graveolens L.) are aromatic medicinal plants belonging to the Apiaceae family. Because of its estrogenic characteristics (chiefly attributed to the main compound anethole), fennel has been used as a galactagogue and emmenagogue, aphrodisiac, uterine tonic compound for thousands of years (22-24). Recent studies have indicated a broad spectrum of pharmacological activities of anise, such as antibacterial (25), antidiabetic, and hypolipidemic (26) effects. Celery has been shown to effectively prevent cardiovascular diseases, lower blood pressure, and strengthen the heart (27).
2. Objectives
Based on ITM and according to recent studies conducted on the estrogenic, antiandrogenic, emmenagogue, and antioxidant effects of fennel, anise, and celery (28), the present study was designed to prepare a suitable formulation of herbal syrup containing these 3 herbs introduced by ITM manuscripts (28), as well as to evaluate the effect of this syrup on letrozole-induced PCOS in female rats.
3. Methods
3.1. Plant Material
Dried fruits of fennel, anise, and celery were purchased from a local market and identified at the Herbarium of Traditional Medicine and Materia Medica Research Center (TMRC), Shahid Beheshti University of Medical Sciences, Tehran, Iran. Herbal Market Samples (HMS) of the fruits of A. graveolens L. (No. 548), P. anisum L. (No. 549), and F. vulgare Mill. (No. 550) have been deposited at the Herbarium of TMRC.
3.2. Chemical, Reagent, and Kits
Letrozole was obtained from Iran Hormone Pharmaceutical Co, Tehran, Iran, and Metformin from Arya Pharmaceutical Co, Tehran, Iran. The other chemicals used in this study consist of Folin-Ciocalteu reagent (Merck, Germany), sodium carbonate (Merck, Germany), gallic acid (Sigma-Aldrich, Germany), and hematoxylin and eosin staining (H&E; Padtanteb, Iran). Serum 17β-estradiol and testosterone levels were measured using ELISA kit KGE014, R&D Systems (Bio-Techne, Minneapolis, MN, USA). Serum progesterone levels were measured using Mouse/Rat Progesterone ELISA kit (Catalog Number SE120087, Sigma-Aldrich, Germany). The serum LH level was evaluated using an ELISA kit (catalog MBS 729873 from MyBioSource, USA) and FSH level using an ELISA kit (catalog MBS 2502190 from MyBioSource, USA).
3.3. Ethical Considerations
The Ethics Committee of Shahid Beheshti University of Medical Sciences approved the proposal of the research (code: IR.SBMU.RETECH.REC.1399.899, 2021). The in vivo part of the study was done according to the NIH Animal Care and Use Committee Guide for the Care and Use of Laboratory Animals (29).
3.4. Formulation Preparation
According to the selected traditional resource (28), our chosen prescription is herbal syrup containing an aqueous extract of anise, fennel, celery seeds, and sugar. Based on the effective and maximum permitted dose of each plant, we made 5 experimental formulations containing 10% plant extract. To prepare the formulations, first, the seeds were crushed, coarsely powdered, and extracted by decoction method with distilled water (plant: water ratio 1: 10 w/v) for 30 minutes. The filtrate was evaporated and concentrated to a final volume (concentration ratio 66: 10). In order to achieve an appropriate viscosity and taste of the formulation, carboxymethylcellulose (CMC) and sugar were added, and various formulations with different ratios of mentioned ingredients (F1 - F5) were prepared. Moreover, sodium benzoate and potassium sorbate were used in the syrup as antimicrobial preservatives.
3.5. Physicochemical Quality Control of the Herbal Syrup
Various physicochemical parameters, including macroscopic and organoleptic characteristics, dried residue, cap locking, density, viscosity, pH, crystallization evaluation, and microbial content, were tested on the final formulation. In addition, the total phenolics content of the herbal syrup was determined by the Folin-Ciocalteau method (30). All tests were performed on 3 batches of the final formulation.
3.5.1. Accelerated Stability Test
According to the International Conference on Harmonization (ICH) Guidelines, the stability study for the final product was completed at accelerated stability conditions (31). Three bottles of syrup were placed at 40 ± 2.0°C in an oven for 6 months. Later, samples were examined every 3 months for the above-mentioned measurements.
3.6. Experimental Protocol
3.6.1. Animals
In this study, 42 healthy female Sprague-Dawley rats aged 6-8 weeks with an average weight of 130 g were prepared from TMRC. Animals were kept in temperature-controlled rooms (22°C), with 45% - 65% humidity and 12-hour light and dark cycles, in a pathogen-free environment. The rats were allowed water and food (standard diet) ad libitum. According to the previous studies (32, 33) and conversion of the human dose to animal dose (34), the treatment groups received doses of 1, 2, 4 mL/kg.
3.6.2. Letrozole Induced-Polycystic Ovarian Syndrome
PCOS was induced by Ndeingang et al.’s method (35). By checking the vaginal smears, the estrous cyclicity of the animals was monitored, and only rats with 2 - 3 regular estrous cycles during the 12 to 14 days of vaginal smear get into the scheme. Group I served as the control group and received 1 mL of 1% CMC (vehicle). Other groups were administered with 1 mg/kg letrozole, dissolved in 1% CMC (2 mL/kg) once daily for 21 days. Vaginal smears were obtained daily to verify the induction of PCOS. Animals were randomly sorted into 6 groups (n = 7 animals in each group). One rat from each group was sacrificed after 21 days of receiving letrozole. Biochemical and histological tests were conducted to confirm PCOS in the rats. From day 22, the animals of group II were gavaged with 30% sugar in distilled water (PCOS group), the animals of group III - V were gavaged with herbal syrup at doses of 1, 2, and 4 mL/kg and served as dose 1, 2, and 3 groups, whereas the animals of group VI were treated with metformin at a dose of 200 mg/kg (36) and considered as the metformin group. Herbal syrup and metformin were given daily for 28 days. At the end of the treatment period, rats were weighed and anesthetized with ketamine/xylazine (5/1 mg/kg) (37). The blood samples were taken for hormonal analysis, and ovaries were collected for histological evaluation.
3.6.3. Serum Hormone Analysis
Blood samples were directly collected from the hearts of the subjects at the end of the experiment. Serum samples were separated by centrifugation and stored at -70°C until use. Hormone levels (FSH, LH, estrogen, testosterone, and progesterone) were measured by enzyme-linked immunosorbent assay (ELISA).
3.6.4. Histological Analysis
On day 49, both ovaries were collected from each animal, quickly removed, cleaned up, and weighed. Later, the ovary was fixed in 10% neutral-buffered formalin for 48 hours. Then, the tissues were embedded in paraffin and cut into 5 μm sections, stained by the H&E staining method. Histological assessment was conducted under light microscopy (Ceti Microscopes, UK). Follicles were identified and scored in different stages: primary, secondary, antral, Graafian, cystic follicles, and corpora lutea.
3.7. Statistical Analysis
The results of the different groups were analyzed by 1-way analysis of variance with the Tukey-Kramer multiple pair comparison test. Statistical difference was considered significant if P < 0.05. Data were displayed as mean ± SEM.
4. Results
4.1. Formulation Preparation and Physicochemical Quality Control
Five experimental formulations were prepared (Table 1). F5 had an acceptable taste and viscosity compared to other formulations and was taken as the final preparation.
| Ingredients (%) | F1 | F2 | F3 | F4 | F5 |
|---|---|---|---|---|---|
| Plant extract | 10 | 10 | 10 | 10 | 10 |
| Sugar | 30 | 30 | 30 | 40 | 50 |
| CMC | 0 | 0.1 | 0.2 | 0 | 0 |
| Sodium benzoate | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Potassium sorbate | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
The results of quality evaluation tests are presented in Table 2. The quality of the syrup was acceptable based on the physical parameters. The microbial count of F5 was within the allowable limits over 6 months, according to the British Pharmacopoeia (38).
| Parameters | Time (mo) | ||
|---|---|---|---|
| 0 | 3 | 6 | |
| Color | Dark brown | Dark brown | Dark brown |
| Taste | Bitter-sweet | Bitter-sweet | Bitter-sweet |
| Appearance | Semi-clear liquid | Semi-clear liquid | Semi-clear liquid |
| Density (g/mL) | 1.24 ± 0 | 1.24 ± 0 | 1.25 ± 0 |
| Viscosity (cP) | 144.4 ± 22.4 | 138.4 ± 15.14 | 286.4 ± 42.80 |
| pH | 5.39 ± 0.03 | 5.38 ± 0.03 | 5.14 ± 0.03 |
| Dried residue (%) | 47.82 ± 0.94 | 49.28 ± 0.87 | 49.58 ± 0.92 |
| Total phenolics content (g/100 mL) (as pyrogallol) | 0.434 ± 0.01 | - | 0.432 ± 0.02 |
a Data are reported as mean ± SD of the mean (n = 3).
4.2. Effects of Herbal Syrup on the Weight of Body and Ovary
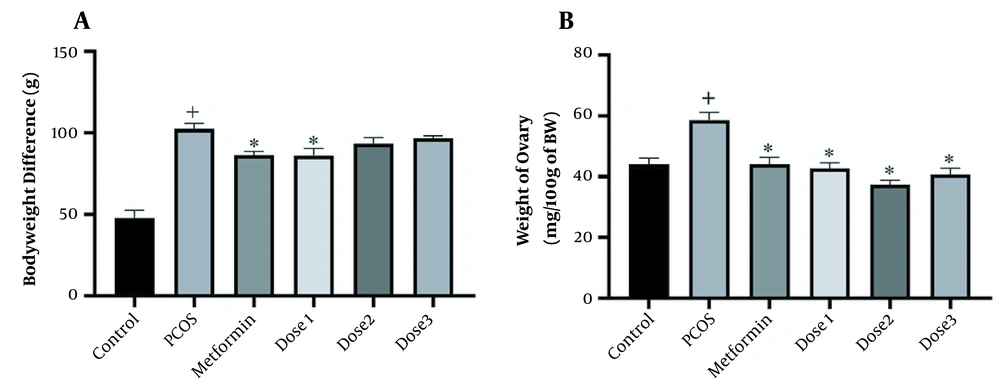
The body weight measurement of the rats had a significant increase before and after treatment in all groups compared to the control group. In contrast, the weight of metformin and dose 1 groups was significantly reduced compared to the PCOS group (Figure 1). Ovaries’ weight significantly increased in the PCOS group compared to the control group and decreased in groups treated with the herbal syrup and metformin compared to the PCOS group. The dose 2 group had the most reduction effect on ovaries weight.
(A) Body weight difference before and after treatment. (B) Ovaries weight measurement. A 1-way analysis of variance followed by Tukey multiple comparisons. Data are expressed as mean ± SEM (n = 6 animals in each group). * P < 0.001 compared to the PCOS group, + P < 0.05 groups compared to the control group.
4.3. Effects of the Herbal Syrup on Hormonal Levels
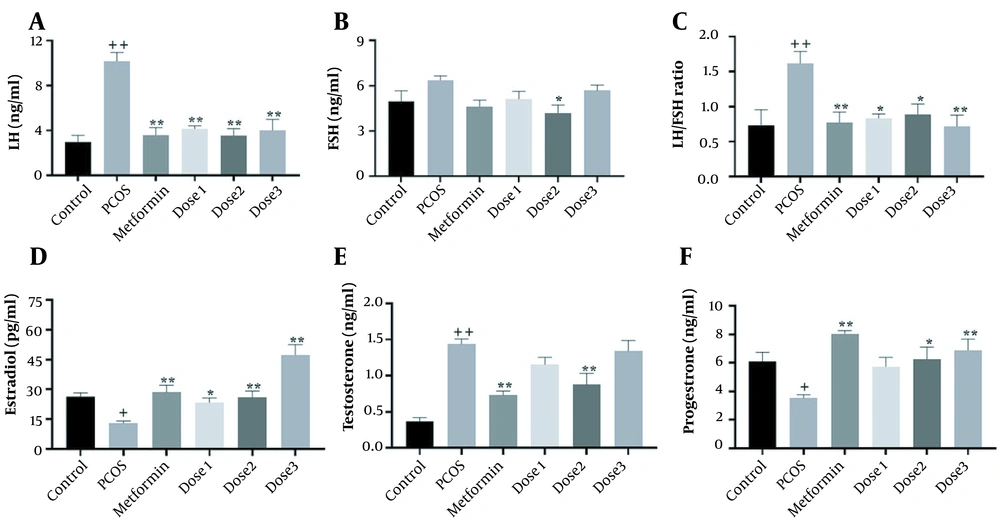
As shown in Figure 2, LH, FSH, LH/FSH ratio, estrogen, testosterone, and progesterone levels were measured in the serum of female rats. In the PCOS group, LH, LH/FSH ratio, and testosterone levels increased (P < 0.001), whereas estradiol and progesterone levels decreased (P < 0.05) compared to the control group. The reverse effect was observed in the metformin group as estradiol and progesterone levels increased. However, LH, LH/FSH ratio, and testosterone levels decreased (P < 0.001) compared to the PCOS group. In all doses of the herbal syrup groups, similar to the metformin groups, LH and testosterone levels decreased. LH levels significantly decreased in all herbal syrup groups, but testosterone reduction was prominent only in the dose 2 group (P < 0.001). In the herbal syrup groups, estradiol and progesterone increased to different levels in a dose-dependent manner. These increases in progesterone levels were not significant in the dose 1 group. There was no significant difference between all groups in FSH levels compared to the control group, and there was a significant reduction in the dose 2 group compared to the PCOS group (P < 0.05).
Mean ± SEM of LH, FSH, testosterone, progesterone, and estradiol concentrations in control, PCOS, metformin, and herbal syrup groups after 28 days of the experiment. (A) LH, (B) FSH, (C) LH/FSH ratio, (D) estradiol, (E) testosterone, and (F) progesterone. Values are expressed as mean ± SEM (n = 6). A 1-way analysis of variance followed by Tukey multiple comparisons. + P < 0.05; + + P < 0.001 as compared to the control group.* P < 0.05; ** P < 0.001 as compared to the PCOS group.
4.4. Effects of the Herbal Syrup on Histology of Ovaries
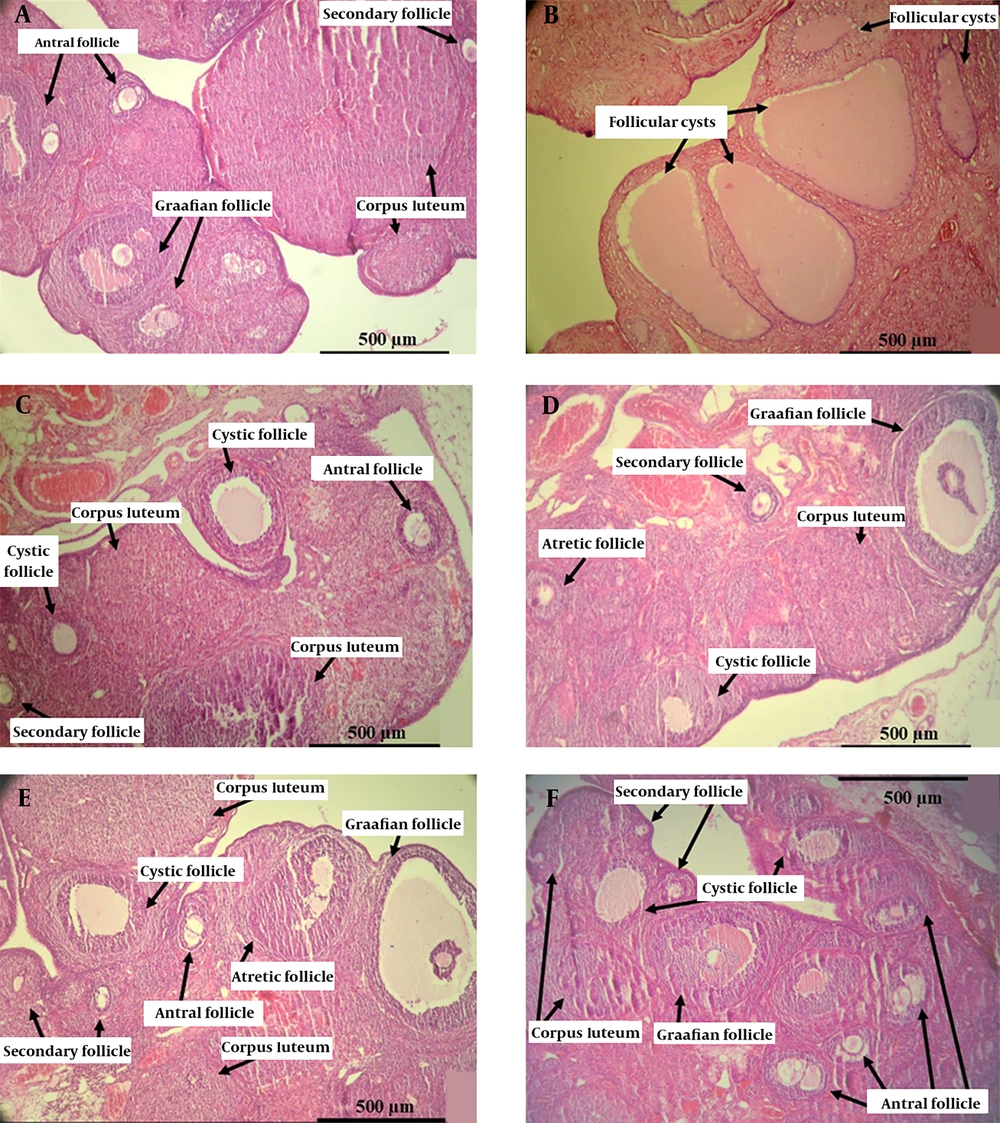
Many large ovarian cystic follicles and fewer corpora lutea were recognized in the PCOS group, whereas no histological abnormalities were observed in the control group. Histological studies of metformin and herbal syrup groups exhibited normal follicular development with a significant improvement in the number of primary, secondary, antral, and Graafian follicles, along with corpora lutea, compared to the PCOS group, which is associated with a significant decrease in the number of cystic follicles (Table 3 and Figure 3).
| Primary Follicle | Secondary Follicle | Antral Follicle | Graafian Follicle | Cystic Follicle | Corpora Lutea | |
|---|---|---|---|---|---|---|
| Control | 6.24 ± 1.17 | 6.13 ± 0.59 | 3.18 ± 0.89 | 1.60 ± 0.80 | 0 | 9.02 ± 1.20 |
| PCOS | 0.50 ± 0.24 a | 3.29 ± 0.33 | 1.47 ± 0.53 | 2.24 ± 0.95 | 13.68 ± 5.84 a | 4.44 ± 0.33 |
| Metformin | 3.25 ± 0.37 b | 5.47 ± 0.88 | 3.69 ± 0.74 | 0.44 ± 0.72 c | 2.33 ± 0.69 c | 11.05 ± 1.11 c |
| Dose 1 | 4.04 ± 0.53 d | 4.44 ± 0.64 | 3.03 ± 0.65 | 0.91 ± 2.57 d | 2.39 ± 2.57 c | 9.75 ± 0.78 b |
| Dose 2 | 4.19 ± 0.46 d | 6.44 ± 0.93 | 4.19 ± 0.69 | 1.11 ± 2.57 b | 3.69 ± 2.57 c | 13.41 ± 1.26 c |
| Dose 3 | 3.16 ± 0.75 b | 8.25 ± 1.62 b | 6.08 ± 1.17 d | 0.66 ± 2.57 c | 3.36 ± 2.57 c | 10.61 ± 1.00 d |
a P < 0.001 as compared to the control group.
b P < 0.05
c P < 0.001 as compared to the PCOS group
d P < 0.01
Photomicrographs of the elected ovarian cross-section from control, PCOS, metformin, and all doses of herbal syrup treated rats after 28 days of the experiment. (A) The control group with different stages of ovarian follicles, including secondary, antral, Graafian follicles, and corpora lutea. (B) The PCOS group with various giant cystic follicles. (C) The metformin group with secondary, antral, Graafian follicles, and corpora lutea. (D) The dose 1 group with small cystic follicles and other follicles. (E) The dose 2 group with mature Graafian follicles, small cystic follicles, other developing follicles, and corpora lutea. (F) The dose 3 group with lots of secondary and antral follicles and small cystic follicles (Hematoxylin and eosin staining, ×10).
5. Discussion
PCOS seems to be a congenital disorder. It is also influenced by environmental factors that cause hormonal changes, including excess androgen production, with clinical features such as irregular menstrual periods, hirsutism, and acne (39). There is no definitive diagnostic test to recognize PCOS, and clinical diagnosis is based on the presence of 3 specific features, oligo-anovulation, androgen excess (either clinical or biochemical), and polycystic ovaries on ultrasound evaluation (40).
Since herbal medicine has long offered suitable therapies for women with irregular menstruation, hyperandrogenism, and PCOS (11), we decided to study the effect of herbal medicine to treat PCOS using ITM. A syrup formulation was prepared based on old prescriptions and current pharmaceutical standards. Fewer additives such as coloring, flavoring, and sweetening agents made the final syrup more similar to the traditional dosage form. In the original formulation of the syrup (28), fennel, anise, and celery seeds were mentioned as effective ingredients, and other excipients, such as sugar and CMC, were used to achieve the desired taste and viscosity.
As shown in Table 1, the F5 formulation with fewer extra ingredients, 10% plant materials, and the best taste and viscosity was chosen as the final preparation.
After 6 months of storage, the F5 samples were preserved their colors, tastes, and semi-clear appearance. Cap locking and crystallization were checked after 1 week, and none of them were observed. The average pH of the samples was 5.30 ± 0.03 at 25°C. The density remained constant, and its average value was 1.24. Additionally, the dried residue was not changed through time. In addition, the phenolic content of the syrup was reduced by less than 5% during the 6 months, which is acceptable according to the ICH protocol (31).
In the next step, the therapeutic effect of the selected formulation was evaluated on PCOS treatment through the animal model. Due to the logistic and ethical limitations, appropriate animal models that mimic many PCOS characteristics have been developed since the 1960s (41). The letrozole induced-PCOS model was introduced in 2003, and in recent years, the number of published studies on the letrozole model has been increasing (42). The letrozole model is similar to the human PCOS in many features such as hormonal imbalance, circulating hyperandrogenism, and ovarian morphologic (43). Letrozole is an inhibitor of the P450 aromatase enzyme that catalyzes the conversion of androgens (androstenedione and testosterone) to estrogens (estrone and estradiol) during steroidogenesis (44). In ovaries, estradiol is generated by converting C19 androgens by aromatase produced by granulosa cells. A decrease in the activity of this enzyme by letrozole could be expected to result in increased ovarian androgen, decreased estrogen, and development of PCOS in the animal models (45). In our study, after 21 days of letrozole administration, PCOS induction was confirmed by irregularities in estrous cycles and an increase in testosterone and LH levels.
One of the main reproductive features of PCOS is hyperandrogenism. The PCOS group showed a significant increase in testosterone levels, presumably reflecting the accumulation of androgens and a significant decrease in estrogen levels induced by enzymatic inhibition of letrozole. Our findings demonstrated that the herbal syrup normalized the hormonal level, and we found the best efficacy at 2 and 4 mL/kg doses. These modifications are probably due to phytoestrogen compounds (such as anethole in fennel, safrole in anise, and apigenin in celery) and antiandrogenic compounds (β-sitosterol and palmitic acid in 3 herbs). It has been revealed that β-sitosterol and palmitic acid (found in fennel, anise, and celery) have antiandrogenic properties and reduce testosterone by inhibiting the dihydrotestosterone-receptor complex (46). Also, estrogenic compounds reduce androgens by stimulating hepatic production of sex hormone-binding globulin (47).
In addition, one of the confirming indicators of PCOS is elevated LH level (35). Excess androgen feedback to the pituitary gland provokes excessive LH and decreases FSH secretion (48). In this study, the herbal syrup was significantly downregulated the LH levels compared to the PCOS group. Besides the antiandrogenic compounds of 3 herbs, chronic use of plant extracts containing phytoestrogens lowers testosterone levels. In the herbal syrup group, probably because of the synergic effect of the 3 herbs, less LH was produced due to the reduction of androgens. A similar study stated that fennel with this mechanism reduced LH levels in PCOS rats (20). In PCOS, the pulse frequency of gonadotropin-releasing hormone (GnRH) increases; therefore, the FSH release decreases in a more significant proportion to LH, and the LH/FSH ratio increases (49). The LH/FSH ratio is typically 1: 1; however, this ratio is 2 or 3 times higher (2: 1 or 3: 1) in PCOS cases (18). A recent study shows that normalizing the LH/FSH ratio is essential in PCOS treatment (50). Furthermore, our results showed that the LH/FSH ratio in PCOS rats was 2.3 times higher than in the control group. This ratio became normal after the herbal syrup administration. Our study does not change FSH levels significantly, similar to previous studies (51, 52). Thus apparently, a decrease in LH levels reduces the LH/FSH ratio. In PCOS women, progesterone blood levels decrease due to anovulation and reduction in the number of corpora lutea (53), and our results agree with these findings. The herbal syrup increased the number of corpora lutea due to lowering LH levels, improving the process of folliculogenesis, and increasing ovulation rates. The corpora lutea secrete the most progesterone and ultimately increase progesterone blood levels. Previous studies have investigated the effects of fennel on improving the hormonal profiles in PCOS and other female disorders, which are consistent with our results (20, 54). Furthermore, the combination of anise and celery regulated menstrual cycles and improved oligomenorrhea in PCOS patients (15). As a standard treatment, metformin improved blood levels of sex hormones significantly, according to the results of the previous study (35).
Obesity is one of the complications that affect PCOS women, with a worldwide prevalence of approximately 35% to 80% (55). The letrozole model was exhibited weight gain in PCOS rats, aligning with our results (43). As an ingredient of this syrup, celery contains a flavonoid named luteolin. Evidence suggests that luteolin suppresses mast cell secretion, especially interleukin (IL)-6, regulates connected adipose tissue angiogenesis, reduces body weight, reduces inflammation in adipose tissue, and lowers insulin resistance (56). The significant weight loss seen in the herbal syrup groups may be due to this combination of celery.
In line with previous studies, our findings indicate that ovarian weight was increased in the PCOS group (48, 57). The herbal syrup and metformin could prevent ovarian weight gain induced by PCOS. This weight loss can be due to a reduction in the number and size of cystic follicles and an improvement in the folliculogenesis process. In PCOS women, hyperandrogenism and elevated LH levels cause multiple cysts to form in the ovaries (58). While LH levels increase, androgens production in the theca cells is accelerated. Subsequently, the relative FSH deficiency reduces the conversion of androgen into estrogen by granulosa cells and disrupts follicle maturation and ovulation (59). In our study, after induction of PCOS, excess androgen production resulted in growth disturbance of antral and Graafian follicles (60). Therefore, the number of different follicles decreased alongside sizeable cystic follicles with a thin granulosa layer. Baravalle et al. showed that letrozole generated follicular dysfunction, such as atretic and large cysts with a thin granulosa layer (61). In our study, the herbal syrup reduced the number of cysts and increased the thickness of the granulosa layer, demonstrating the improvement of ovarian tissue in these groups. The herbal syrup improved folliculogenesis and increased the number of primary, secondary, antral, and Graafian follicles. These increases have also been reported after fennel administration due to estrogenic properties (62). Besides, the antioxidant features of fennel, anise, and celery could effectively prevent ovarian follicle atresia by diminishing oxidative stress (61, 63).
5.1. Conclusions
Our study exhibited that the herbal syrup (containing fennel, anise, and celery) could recover ovarian morphology and upregulate serum levels of progesterone and testosterone. In contrast, it could decrease LH, estradiol, and FSH serum levels in the letrozole-induced PCOS model. The studied herbal syrup might be a potent therapeutic herbal drug for PCOS treatment. Further clinical investigations are required to confirm the potential therapeutic effects of the recently studied herbal syrup.