Abstract
Background:
Pinus eldarica contains various components such as phenolic and terpene materials. Previous reports showed these two components had anti-insect properties. Pest control, especially for different species of cockroaches, is important because of transmission of various diseases.Objectives:
The aim of this study was to determine the anti-insect potential of P. eldarica leaf essential oil (PLE) against Blattella germanica, German cockroach.Methods:
The anti-insect activity of different doses (50, 150, and 300 μg/mL) of PLE of two areas of clean air (CA) zones and polluted air (PA) zones was evaluated by contact toxicity, fumigant toxicity, and repellence tests.Results:
The results of contact test showed that the percentages of mortality rates were increased significantly in a dose- and time-dependent manner in both contact and fumigation methods as well as repellency percentage. Also, the results related to PA zones were more significant than CA zones.Conclusions:
This study showed that essential leaf oil of P. eldarica induced mortality and repellency in German cockroach, and it can probably be used as a good candidate for pest control, in particular cockroaches.Keywords
Pinus eldarica Blattella germanica Insecticide Effect Repellent Effect
1. Background
German cockroach, with the scientific name Blattella germanica, is a domestic pest that resides in the places of human life such as houses, hotels, dormitories even in related-health sections like hospitals (1). This insect, as a biological threat, carries different pathogens, e.g., fungi, viruses, and bacteria (2). Cockroach can be considered a health problem worldwide. One of the common methods of controlling this pest is the use of chemical insecticides for example, phosphorus, carbamate, pyrethroids components, piperonyl butoxide, and s,s,s-tributyl phosphorotrithioate, etc. (3). However, long-term use, overexposure, and useless use lead to resistance in German cockroach against them (4). So, researchers are trying to find new structures with low toxicity for environment and biodegradation (5). One of these candidates is plants that have inherent and natural characteristics for fighting against insects. Pinus eldarica, as Iranian kind of pine trees, has antioxidant properties due to its containing phenol and fatty acids that are used as a food and medicine supplement (6). In several countries, P. eldarica is a member of pinaceae family and is located widely in Iran, Pakistan, and Afghanistan (7). In traditional medicine, different parts (needles, resin, buds, and nuts) of this plant have been used for various disorders such as bronchial asthma, allergic rashes, skin wounds, dermatitis, skin irritations, and diabetes (8). Pinus eldarica has several compounds like α-pinene, β-caryophyllene, longifolene, δ-3-carene, β-pinene, and polyphenols materials, including catechin, caffeic acid, ferulic acid, quercetin, abietic acid myrcene, camphene, and taxifolin (6, 9). Previous studies on the oil of its fruits indicated the major components are made up of β-pinene (3.8%), δ-3-carene (6.3%), α-humulene (6.4%), longifolene (10.5%), α-pinene (16.3%), β-caryophyllene (34%) (24.3). Kurdelas et al. reported that δ-3-carene and α-pinene had repellent effects against mosquito species (10). Moreover, α-pinene of Pistacia atlantica subsp. kurdica had insecticidal activities against different insect species (11).
2. Methods
2.1. Plant Materials
Leaves of P. eldarica were collected from two zones of Isfahan city, center of Iran, in Sep 2018, including clean air (CA) zones from near mountains and polluted air (PA) zones from center of town where it has high industrial pollution. Taxonomic determination was approved by department of Biology at Islamic Azad University, Falavarjan Branch, Falavarjan, Iran.
2.2. Preparation of Aqueous Extract
The collected leaves of P. eldarica were placed in a dark-room for drying process. Then, they were powdered and fruit extraction was obtained by Clevenger methods as described previously (12, 13). Briefly, one percent of fruit powder was added to 1,000 mL of distilled water (hot) for half an hour and filtered by Whatman filter papers (11 μm pore size, No. 1). Finally, the solvent was removed by using rotary evaporator in suitable condition was removed and then P. eldarica leaf essential oil (PLE) was stored at -20°C until initiation of experiments.
2.3. Insects
Groups of B. germanica were chosen randomly from the Lab-animal Center of Falavarjan Branch and housed in glass jars with 12/12 hours of artificial day/night cycle at room temperature (25 ± 1.5).
2.4. Laboratory Bioassays
2.4.1. Contact Toxicity
For testing toxicity of PLE as described previously by Appel et al. (2) briefly, different concentrations of PLE were prepared as 50, 150, and 300 μg/mL in acetone solvent. Acetone alone was used for treatment of the control group. Then, 2.5 mL of appropriate concentrations (50, 150, and 300 μg/mL) were added to Whatman filter that was placed in glass jars. After solvent evaporation for 15 min, the cockroaches entered the glass jars. Each group consisted of ten cockroaches in every experiment and each test was repeated at least three times. Mortality rate of this test was evaluated after 5, 15, and 30 min by light exposure and touching their bodies by applicators and paying attention to their motions. Lack of movement was considered a dead cockroach. This experiment was done separately for both CA zones and PA zones extracts.
2.4.2. Fumigant Toxicity
Appel et al. (2) investigation was used as a reference for testing fumigant activity. Briefly, fumigant toxicity of PLE was calculated by placing ten insects in a glass jar with 1.5 cm diameter cotton roll that was treated with 100 μL of different concentrations of PLE (50, 150, 300 μg/mL). Acetone was used for the control group lonely. To prevent the insects from contacting directly to extract, we injected each concentration in the center of the cotton rolls. After 5, 15, and 30 min, the numbers of dead cockroaches were calculated as previously described in contact test. The experiment was repeated at least three times. As explained, this investigation was done for both extracts separately (CA zones and PA zones).
2.4.3. Repellency Test
This test was adopted from the research done by Ferrero et al. (14). In brief, each Whatman filter was divided into two halves, one was treated with 1.5 mL of acetone, and another half was exposed with 1.5 mL of the different concentration of PLE (50, 150, 300 μg/mL) after 15 min for solvent evaporation, the filter papers were fitted together to make a single layer and used to cover the floor of a glass jar. Similar Whatman filter with two halves, including: (1) treated with acetone; and (2) untreated used for the control group. Cockroaches (10 Num.) were released in the center of jars, and after five hours, their distribution was recorded. Each experiment was done in triplicate. Finally, the formula (RV = T/NT) was used to determine the rate of repellent effect of PLE that RV = repellency value; T = Num. of cockroaches on treated zone; NT = Num. of cockroaches on untreated zone. The experiment was done for both extracted plants from CA zones and PA zones.
2.5. Statistical Analysis
Data were analyzed with SPSS software and one-way ANOVA, Duncan test.
3. Results
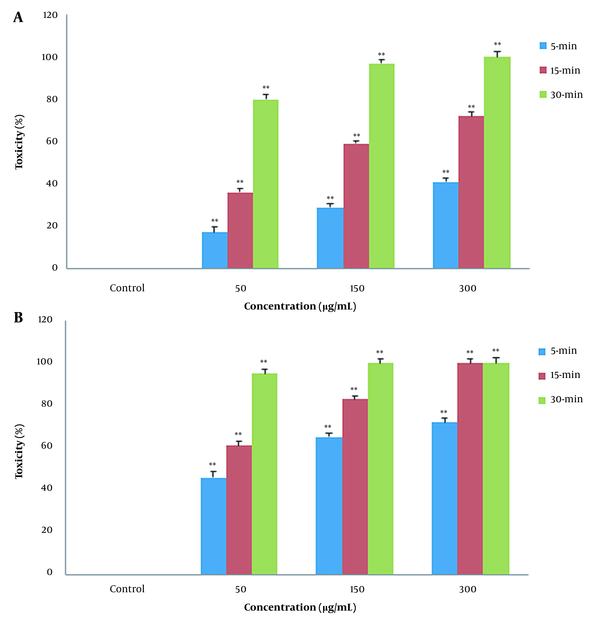
In contact test, the effects of PLE on the B. germanica were examined. The insects were exposed to different concentrations (50, 150, 300 μg/mL) of PLE for 5, 15, and 30 min. Afterward, cockroaches’ mortality rate was measured. As Figure 1A shows, PLE of CA zones demonstrated a significant increase in time- and concentration-dependent manner in mortality rate (P < 0.01). This tendency, even with more severity, was shown in Figure 1B due to effect of PLE of PA zones on the cockroaches’ mortality rate (P < 0.01). The plant extracts from PA zones significantly increased cockroaches’ mortality rate in comparison to the plant extracts from CA zones in contact toxicity (Figure 1A and B).
In contact toxicity manner, the effect of Pinus eldarica leaf essential oil (PLE) is shown on percentage mortality rate of Blattella germanica. The B. germanica were exposed to different concentrations (50, 150, and 300 μg/mL) of PLE for 5, 15, and 30 min, and their mortality rate was measured. Pinus eldarica leaf essential oil increased the mortality rate in B. germanica in a time- and concentration-dependent manner in both extracted plants in A, clean-air zones; and B, polluted-air zones. Each value is presented as mean ± SD of at least three experiments (triplicate). Error bars represent standard deviation. * P < 0.05, ** P < 0.01 are significant compared to unexposed B. germanica as the control groups.
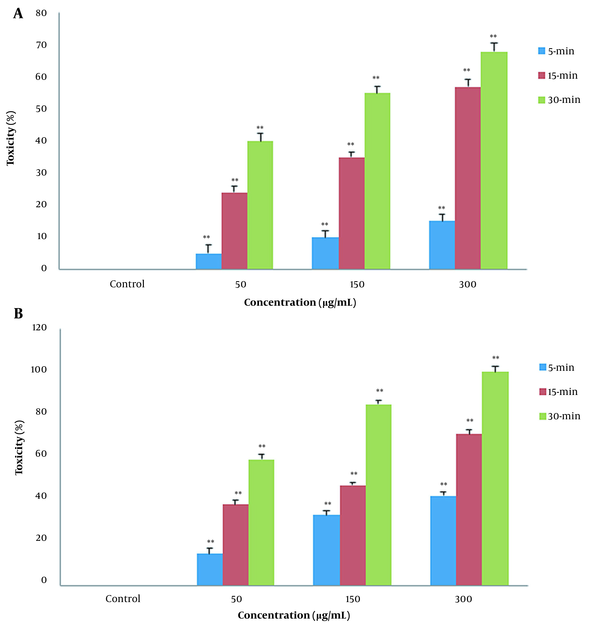
In fumigant toxicity manner, the effect of PLE on the mortality of the B. germanica was tested in a time concentration-dependent manner. Our finding showed that the percentages of mortality significantly increased in both CA zones and PA zones extracts and the percentage of deaths in PA compared to CA increased gradually (Figure 2). The results of comparison of fumigant toxicity vs. contact toxicity in PLE of CA zones are shown in Figure 3.
In fumigant toxicity manner, the effect of Pinus eldarica leaf essential oil (PLE) is indicated on percentage mortality rate of Blattella germanica. The B. germanica were exposed to different concentrations (50, 150, and 300 μg/mL) of PLE for 5, 15, and 30 min, and their mortality rates were measured. Pinus eldarica leaf essential oil increased mortality rate in B. germanica in a time- and concentration-dependent manner in both extracted plants in A, clean-air zones; and B, polluted-air zones. Each value is presented as mean ± SD of at least three experiments (triplicate). Error bars represent standard deviation. * P < 0.05, ** P < 0.01 are significant compared to unexposed B. germanica as the control group.
Effect of Pinus eldarica leaf essential oil of clean-air zones on percentage mortality rate in the Blattella germanica in respect of contact toxicity vs. fumigant toxicity. * P < 0.05 and ** P < 0.001 contact toxicity compared to fumigant toxicity.
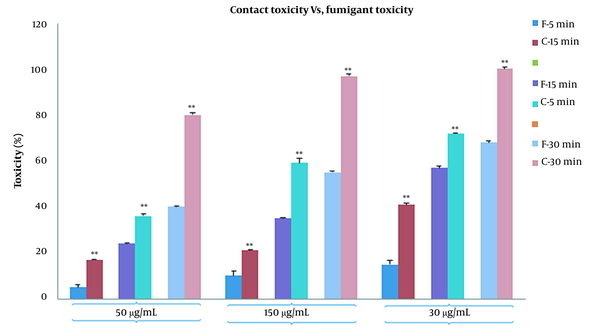
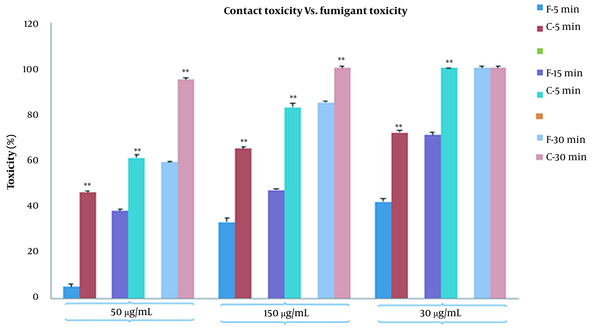
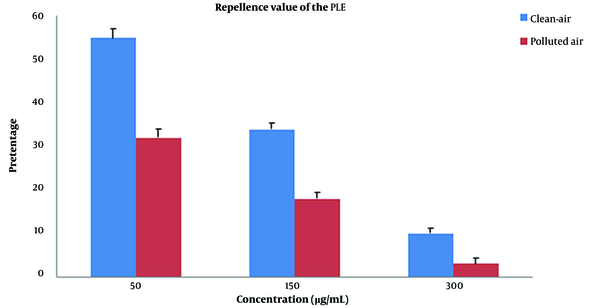
It showed that 50 μg/mL concentration was statistically more significant in fumigant toxicity than contact toxicity. This tendency also existed in other concentrations and times. The results of the comparison of the mortality rate between fumigant toxicity and contact toxicity of PLE of PA zones are shown in Figure 4. The percentage of the mortality rate of PLE in fumigant test was more than contact test at all times and concentrations except for 30-min analysis in 300 μg/mL. Repellent values of PLE are shown in Figure 5. As shown, the minimum repellency was shown in 50 μg/mL concentration, whereas the highest repellency was observed in 300 μg/mL concentration of B. germanica against after a 5-hour interval, and PLE of PA zones were more than PLE of CA zones.
Effect of Pinus eldarica leaf essential oil of polluted-air zones on percentage mortality rate in the Blattella germanica in respect of contact toxicity vs. fumigant toxicity. * P < 0.05; ** P < 0.001 contact toxicity compared to fumigant toxicity.
Repellence value of the Pinus eldarica leaf essential oil (PLE) in Blattella germanica following the PLE treatment in a concentration-dependent manner after five hours. Error bars represent standard deviation. * P < 0.05; ** P < 0.001. Polluted-air extract vs. clean-air extract
4. Discussion
Chemical agents are common methods for insect control, in particular, cockroaches; however, nowadays, several reasons, such as bad effects on human health and resistance against anti-insects by cockroaches made researchers find alternative agents (15, 16). One of the basic cases is agents with natural origin. So, the present study aimed to evaluate the impact of PLE on the mortality rate of cockroaches.
The P. eldarica has therapeutic properties in traditional medicines. Recent studies showed that P. eldarica has beneficial components with different characteristics such as antioxidant, antineoplastic, anti-inflammation, and immune modulatory effects through nitric oxide, prostaglandin E-2, regulation of cyclooxygenase and cancer-related proteins (17-19). A previous report showed the fruits of this plant led to prevention of kidney stones, particularly calcium oxalate (8). Also, different extracts of plant have antibacterial features against Pseudomonas aeruginosa and Escherichia coli strains (3, 20). Phytochemical analysis on P. eldarica showed that it is rich in different phenolic components like taxifolin, vanillic acid, coumaric acid, gallic acid, epicatechin, catechin, ferulic acid, and caffeic acid. As well, other components such as δ-3-carene, α-humulene, β-caryophyllene, longifolene, α- and β-pinene some of which had anti-insect properties (7, 21-23). To the best of our knowledge, this is the first study that aimed to evaluate possible anti-insect effects of fruits of P. eldarica on B. germanica. In the current study, the anti-insect activities of PLE of CA zones and PA zones were investigated on B. germanica.
The findings showed in contact toxicity test, the percentages of mortality rate of PLE on the B. germanica statistically increased in both extracted fruits of CA zones and PA zones, but this effect in PA zones was dramatically more than CA zones. In fumigant toxicity manner, the mortality rate had an increased trend in time- and concentration-dependent manner. In this case, as well, plant essential oil from PA zones was more effective in the mortality rate of cockroaches compared with CA zones. The comparison between contact toxicity and fumigant toxicity showed that the percentage of mortality rate due to contact toxicity was significantly more than fumigant toxicity in both zones. Investigation on repellence value of PLE showed that high repellency in a concentration-dependent manner after five hours and as predictable, extracted fruit from PA zones had more repellence value than extracted fruits from CA zones.
Regarding the effect of pollution on the components of plants, several reasons were observed that pollution could induce tension in plants, thereby increasing some materials, such as terpene and secondary metabolites (24). Candan et al., in 2003, found that secondary metabolites led to an increase in antioxidant properties of a plant (25). Arastegan and Amjad, in 2015, showed that extracted fruits of P. eldarica in PA zones had more terpene components like α-pinene, β-caryophyllene, δ-3-carene, and β–pinene than extracted fruits of P. eldarica in CA zones (26). Thus, pollution leads to high production of terpene components, that these materials are one of the important anti-insect agents (26). In a study done by Sharifi-fard et al., they found that extract of Eucalyptus sp. led to entire mortality in Supella longipalpa F at %5 concentration after 26 hours, while they did not observe any mortality in the control group (27). Zandi and Ramazani mentioned that extraction of both Mentha arvensis and Mentha pulegium led to enhance in the mortality rate of Callosobruchus maculatus (28). In a study concerning the impact of three essential oils, Cymbopogen citratus, M. arvensis, Eucalyptus citriodora, against Periplaneta americanca, Farkhanda showed C. citratus had 20 - 100% toxicity and 70 - 100% toxicity in contact and fumigant tests after 24 hours, respectively and high repellency against others (29). Calmasur et al., in 2006, showed three plants had high toxicity against adults of Bemisia tabaci, nymphs, and adults of Tetranychus urticae Koch in time- and concentration-dependent manner like the present study (30).
Alshehry et al., in 2014, found that extract of four plants (Rhazya stricta Decne, Lantana camara L., Ruta chalepensis L., and Heliotropium bacciferum) had remarkable toxicities against subterranean termites and Psammotermes hybostoma in time- and concentration-dependent manner (31). The power of five local insecticides (cypermethrin EC10%, deltamethrin EC5%, lambda-cyhalothrin EC5%, diazinon EC0.5%, and Negon® (permethrin + propoxur oil liquid1%) commercial formulations) against B. germanica was investigated by Shahi et al. who showed that cypermethrin insecticide caused maximum mortality rates of 20, 35, 90 and 100% in B. germanica after one hour of contact (4). The present findings showed that an aqueous extract of P. eldarica could induce mortality in B. germanica in both types, extracted from CA zones and PA zones. These findings provide evidence that P. eldarica may potentially be used as an anti-insect agent.
References
-
1.
Arruda LK, Vailes LD, Ferriani VP, Santos AB, Pomes A, Chapman MD. Cockroach allergens and asthma. J Allergy Clin Immunol. 2001;107(3):419-28. [PubMed ID: 11240940]. https://doi.org/10.1067/mai.2001.112854.
-
2.
Appel AG, Gehret MJ, Tanley MJ. Repellency and toxicity of mint oil to American and German cockroaches (Dictyoptera: Blattidae and Blattellidae). J Agric Urban Entomol. 2001;18(3):149-56.
-
3.
Ostad SN, Vazirian M, Pahlevani R, Hadjiakhondi A, Hamedani MP, Almasian A, et al. Toxicity evaluation of aromatic water of Pinus eldarica Medw. in acute and sub-chronic toxicity experiments. Prog Nutr. 2018;20:68-74.
-
4.
Shahi M, Hanafi-Bojd AA, Vatandoost H. Evaluation of five local formulated insecticides against German cockroach (Blattella germanica L.) in Southern Iran. Iranian J Arthropod-Borne Dis. 2008;2(1):21-7.
-
5.
Pascual-Villalobos MJ, Robledo A. Screening for anti-insect activity in Mediterranean plants. Ind Crops Prod. 1998;8(3):183-94. https://doi.org/10.1016/s0926-6690(98)00002-8.
-
6.
Mehrzadi S, Ghaznavi H, Tajallizadehkhoob Y, Fakhrzadeh H. Effects of Pinus eldarica Medw. nut extract on blood glucose and cholesterol levels in hypercholesterolemic alloxan-induced diabetic rats. J Medicinal Plants. 2013;12(45):68-74.
-
7.
Afsharypuor S, San'aty F. Essential Oil Constituents of Leaves and Fruits of Pinus eldarica Medw. J Essent Oil Res. 2005;17(3):327-8. https://doi.org/10.1080/10412905.2005.9698920.
-
8.
Hosseinzadeh H, Khooei AR, Khashayarmanesh Z, Motamed-Shariaty V. Antiurolithiatic activity of Pinus eldarica medw: fruits aqueous extract in rats. Urol J. 2010;7(4):232-7. [PubMed ID: 21170851].
-
9.
Ghadirkhomi A, Safaeian L, Zolfaghari B, Agha Ghazvini MR, Rezaei P. Evaluation of acute and sub-acute toxicity of Pinus eldarica bark extract in Wistar rats. Avicenna J Phytomed. 2016;6(5):558-66. [PubMed ID: 27761426]. [PubMed Central ID: PMC5052419].
-
10.
Kurdelas RR, López S, Lima B, Feresin GE, Zygadlo J, Zacchino S, et al. Chemical composition, anti-insect and antimicrobial activity of Baccharis darwinii essential oil from Argentina, Patagonia. Ind Crops Prod. 2012;40:261-7. https://doi.org/10.1016/j.indcrop.2012.03.024.
-
11.
Sadeghi A, Pourya M, Smagghe G. Insecticidal activity and composition of essential oils from Pistacia atlantica subsp. kurdica against the model and stored product pest beetle Tribolium castaneum. Phytoparasitica. 2016;44(5):601-7. https://doi.org/10.1007/s12600-016-0551-0.
-
12.
Galavi HR, Saravani R, Shahraki A, Ashtiani M. Anti-proliferative and apoptosis inducing potential of hydroalcoholic Achillea wilhelmsii C. Koch extract on human breast adenocarcinoma cell lines MCF-7 and MDA-Mb-468. Pak J Pharm Sci. 2016;29(6 Suppl):2397-403. [PubMed ID: 28167484].
-
13.
Saravani R, Galavi HR, Shahraki A. Inhibition of Phosphodiesterase 5 and Increasing the Level of Cyclic Guanosine 3',5' Monophosphate by Hydroalcoholic Achillea wilhelmsii C. Koch Extract in Human Breast Cancer Cell Lines MCF-7 and MDA-Mb-468. Breast Cancer (Auckl). 2017;11:1178223417690180. [PubMed ID: 28469435]. [PubMed Central ID: PMC5391053]. https://doi.org/10.1177/1178223417690178.
-
14.
Ferrero AA, Chopa CS, Gonzalez JO, Alzogaray RA. Repellence and toxicity of Schinus molle extracts on Blattella germanica. Fitoterapia. 2007;78(4):311-4. [PubMed ID: 17490831]. https://doi.org/10.1016/j.fitote.2006.11.021.
-
15.
Jang YS, Choi DS, Ahn YJ. Vapor Phase Toxicity to Insecticidal and Repellent Properties of Nine Volatile Constituents of Essential Oils Against the American Cockroach Periplaneta americana (L.). Pestic Sci. 2005;54(3):261-8.
-
16.
Thavara U, Tawatsin A, Bhakdeenuan P, Wongsinkongman P, Boonruad T, Bansiddhi J, et al. Repellent activity of essential oils against cockroaches (Dictyoptera: Blattidae, Blattellidae, and Blaberidae) in Thailand. Southeast Asian J Trop Med Public Health. 2007;38(4):663-73. [PubMed ID: 17883004].
-
17.
Bolandghamat S, Moghimi A, Iranshahi M. Effects of ethanolic extract of pine needles (Pinus eldarica Medw.) on reserpine-induced depression-like behavior in male Wistar rats. Pharmacogn Mag. 2011;7(27):248-53. [PubMed ID: 21969797]. [PubMed Central ID: PMC3173901]. https://doi.org/10.4103/0973-1296.84240.
-
18.
Potta S, Doss MX, Hescheler J, Sachinidis A. Epigallocatechin-3-Gallate (EGCG): A Structural Target for the Development of Potential Therapeutic Drugs Against Anti-Proliferative Diseases. Drug Design Reviews - Online. 2005;2(1):85-91. https://doi.org/10.2174/1567269053390329.
-
19.
Karonen M, Hamalainen M, Nieminen R, Klika KD, Loponen J, Ovcharenko VV, et al. Phenolic extractives from the bark of Pinus sylvestris L. and their effects on inflammatory mediators nitric oxide and prostaglandin E2. J Agric Food Chem. 2004;52(25):7532-40. [PubMed ID: 15675800]. https://doi.org/10.1021/jf048948q.
-
20.
Sadeghi M, Zolfaghari B, Jahanian-Najafabadi A, Abtahi SR. Anti-pseudomonas activity of essential oil, total extract, and proanthocyanidins of Pinus eldarica Medw. bark. Res Pharm Sci. 2016;11(1):58-64. [PubMed ID: 27051433]. [PubMed Central ID: PMC4794938].
-
21.
Zolfaghari B, Iravani S. Essential Oil Constituents of the Bark ofPinus pinasterfrom Iran. J Essent Oil-Bear Plants. 2012;15(3):348-51. https://doi.org/10.1080/0972060x.2012.10644057.
-
22.
Sadeghi AM, Fallah HH, Tajalizadekhoob Y, Mirarefin M, Taheri E, Saeednia S, et al. Determination of phenolic compounds in Pinus eldarica by HPLC. J Medicinal Plants. 2014;13(49):22-33.
-
23.
Iravani S, Zolfaghari B. Phytochemical analysis of Pinus eldarica bark. Res Pharm Sci. 2014;9(4):243-50. [PubMed ID: 25657795]. [PubMed Central ID: PMC4314872].
-
24.
Papiez MR, Potosnak MJ, Goliff WS, Guenther AB, Matsunaga SN, Stockwell WR. The impacts of reactive terpene emissions from plants on air quality in Las Vegas, Nevada. Atmos Environ. 2009;43(27):4109-23. https://doi.org/10.1016/j.atmosenv.2009.05.048.
-
25.
Candan F, Unlu M, Tepe B, Daferera D, Polissiou M, Sokmen A, et al. Antioxidant and antimicrobial activity of the essential oil and methanol extracts of Achillea millefolium subsp. millefolium Afan. (Asteraceae). J Ethnopharmacol. 2003;87(2-3):215-20. [PubMed ID: 12860311]. https://doi.org/10.1016/s0378-8741(03)00149-1.
-
26.
Arastegan N, Amjad L. Evaluation of environmental pollutions on Pinus eldarica needles essential oil. J Bio & Env Sci. 2015;7(1):134-40.
-
27.
Sharifi-fard SF, Siahpoush A, Kasiri H. [Insecticide and Dehumidifying Properties of Eucalyptus Extract in Control of Brown Banded Cockroach (the Most Important Factor for Tropical and Infectious Diseases) in Hospitals and Residential Areas]. IJIDTM Journal. 2013;1(64):67-73. Persian.
-
28.
Zandi SN, Ramazani L. [Investigation of Insecticidal Activity of Essential Oil of Mentha arvensis and Pune Mentha pulegium on Callosobruchus maculatu]. Plant Protection (Scientific Journal Of Agriculture). 2012;35(2):1-11. Persian.
-
29.
Farkhanda M. Efficacy of some essential oils against American cockroach Periplaneta americana (L.). J Med Plant Res. 2012;6(6). https://doi.org/10.5897/jmpr11.1244.
-
30.
Çalmaşur Ö, Aslan İ, Şahin F. Insecticidal and acaricidal effect of three Lamiaceae plant essential oils against Tetranychus urticae Koch and Bemisia tabaci Genn. Ind Crops Prod. 2006;23(2):140-6. https://doi.org/10.1016/j.indcrop.2005.05.003.
-
31.
Alshehry AZ, Zaitoun AA, Abo-Hassan RA. Insecticidal activities of some plant extracts against subterranean termites, Psammotermes hybostoma (Desneux)(Isoptera: Rhinotermitidae). Int J Agric Sci. 2014;4(9):257-60.