1. Background
In recent decades, cancer incidence and mortality rates have witnessed a rapid global increase. Malignant melanoma, an aggressively deadly form of skin cancer, ranks among the cancers with the highest mortality rates (1). The number of diagnosed melanoma cases has been doubling every 10 to 20 years due to both population growth and aging. Several environmental and genetic risk factors contribute to melanoma, including ultraviolet (UV) exposure, environmental and occupational hazards, immunosuppression, the presence of atypical nevi, and genetic and epigenetic factors (2, 3). Despite the availability of therapeutic options, such as surgical resection, biochemotherapy, photodynamic therapy, immunotherapy, radiation therapy, cytotoxic chemotherapy, and gene therapy, the response to treatment in advanced melanoma cases often remains poor due to its metastatic nature (4). Moreover, melanoma commonly exhibits resistance to conventional treatments, such as drug therapy and chemo-radiation, underscoring the need for the development of new, cost-effective, and low-risk treatment approaches (5).
The Allium genus, a member of the Amaryllidaceae family, encompasses more than 850 diverse species. Among its most well-known members are garlic, onions, shallots, chives, and leeks. These herbs, steeped in ancient traditions, play a significant role in preventing and treating various ailments, including diabetes, headaches, hemorrhages, atherosclerosis, and inflammatory diseases (6, 7). Owing to their rich composition of important compounds, such as polyphenols, saponins, and sulfur compounds, Allium species have recently garnered attention as promising agents for cancer prevention and supportive therapy. The evidence from various studies suggests that the phytochemicals present in Allium plants hold the potential to prevent and inhibit cancer progression. Additionally, combining Allium extracts with chemotherapy has yielded promising clinical outcomes while remaining cost-effective (8-10).
Allium jesdianum, an onion-like plant indigenous to the western and southwestern highlands of Iran, is known by local names, such as Bowser, Sarpa, or Bonsorkh, owing to the reddish hue of its basal leaf parts. A. jesdianum has been found to possess a range of medicinal properties, including analgesic effects, prevention of platelet aggregation, kidney stone formation inhibition, and even anti-Alzheimer activity (11, 12). Various studies have demonstrated the anticancer potential of A. jesdianum against human colon cancer cells, cervical cancer cells, myelogenous leukemia, and thyroid cancer cells (13-15). Despite its widespread use in traditional medicine and its known anticancer properties, there has been no investigation into the effects of this native plant on melanoma until now.
2. Objectives
The current study examined the anticancer properties of the hydroalcoholic extract of A. jesdianum on human malignant melanoma cells by evaluating cell viability, cytotoxic activities, reactive oxygen species (ROS) formation, lipid peroxidation, and the release of cytochrome c (CYT-C).
3. Methods
3.1. Preparation of Plant Sample
The A. jesdianum collected in spring from the Bakhtiari Mountains was coded (A-0138) by a relevant expert at Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran. The dried plant extract was accumulated using the maceration technique using 80:20 ethanol: water solvent and kept in the refrigerator (13).
3.1.1. Total Phenolic Compounds
The total phenolic content of the hydroalcoholic extract of A. jesdianum was measured according to the Folin-Ciocalteu assay described previously (16).
3.1.2. Flavonoid Compounds
The total flavonoid content was determined using the aluminum chloride assay, with catechin serving as the standard for calculating the total flavonoid content in the samples. The results were expressed as milligrams of catechin equivalent per 100 g of the sample (mg CE/100 g sample). All the samples underwent triplicate analysis (mg/g) (17).
3.2. Cell Culture
The human malignant melanoma cell line, A375, and the normal human fibroblast cell line, AGO-1522, were procured from the Iranian Biological Resource Center (Tehran, Iran). In this study, AGO-1522 was utilized as a control for comparative analysis (18).
3.3. Cytotoxicity
When investigating new anticancer agents, the first parameter assessed is their antiproliferative effect. To assess this effect, a colorimetric method employing 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide (MTT) was employed after 24 hours. The cell viability of A375 and AGO-1522 was measured following treatment with A. jesdianum. The half maximal inhibitory concentration (IC50) value, indicating the concentration at which 50% of the cells cannot survive and cease to proliferate, was determined using the probit regression model in the PRISM program. Subsequently, the cytotoxic activity of A. jesdianum extract was compared between A375 and AGO-1522 using the MTT method (19).
3.4. Lipid Peroxidation
Thiobarbituric acid-reactive substances (TBARS), by-products of lipid peroxidation, were assessed spectrophotometrically (20).
3.5. Reactive Oxygen Species
The fluorometric technique was employed to determine the accumulation of intracellular ROS. A total of 2500 cells were cultured in 96-well plates and incubated for 24 hours. Before incubation, the cells were treated with an extract derived from A. jesdianum for 24 hours. Subsequently, the cells were washed twice with phosphate-buffered saline (PBS) and incubated at 37°C with diacetyldichlorofluorescein (DCFH-DA) at a concentration of 10 µM for 30 minutes. The fluorescence intensity of dichlorofluorescein (DCF) was measured at an excitation wavelength of 485 nm and an emission wavelength of 528 nm (21).
3.6. Cytochrome c Release
An immunoassay kit from R & D Systems, Inc. (Minneapolis, Minnesota, USA) was utilized for the quantitative determination of CYT-C concentrations. By using a spectrophotometer set to 450 nm, the optical density of each well was determined (20).
3.7. Statistical Analysis
The assays were performed three times in replicates, and the mean value was used for statistical analysis. The results were expressed as mean ± standard deviation (SD). To assess the statistical significance, a one-way analysis of variance (ANOVA) test followed by the post hoc Tukey test was applied. The significance level was set at P < 0.05.
4. Results
4.1. Extract Standardization
Based on the study, the hydroalcoholic extract of A. jesdianum contained 157.6 mg/g of dry extract based on gallic acid, and the amount of flavonoids was 114.7 mg/1 mg/g of dry extract based on quercetin.
4.2. Cytotoxicity
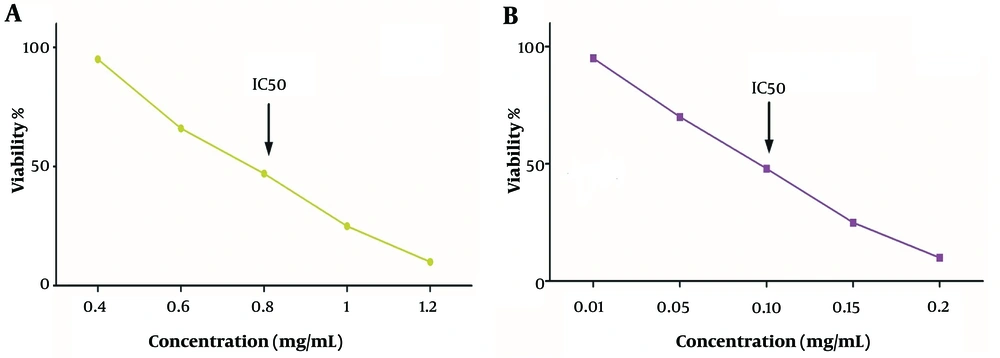
The A375 and AGO-1522 cell lines exhibited different responses when exposed to the A. jesdianum extract in a dose-dependent manner. The IC50 value, representing the concentration causing 50% cell inhibition, was determined by analyzing the graph depicting the percentage of inhibition against various extract concentrations. The IC50 values for A375 and AGO-1522 were 0.120 and 0.819 mg/mL, respectively. Consequently, all the experiments were conducted at 0.1 mg/ml (Figure 1).
Determination of half maximal inhibitory concentration (IC50) values of Allium jesdianum extract on normal fibroblast cell line (AGO-1522) (A375) (A) and human melanoma cell lines (B) during 24 hours. Cytotoxicity was measured using MTT dye. The IC50 value indicates the concentration at which 50% of the cells are unable to survive and cease to proliferate.
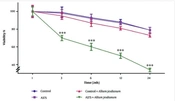
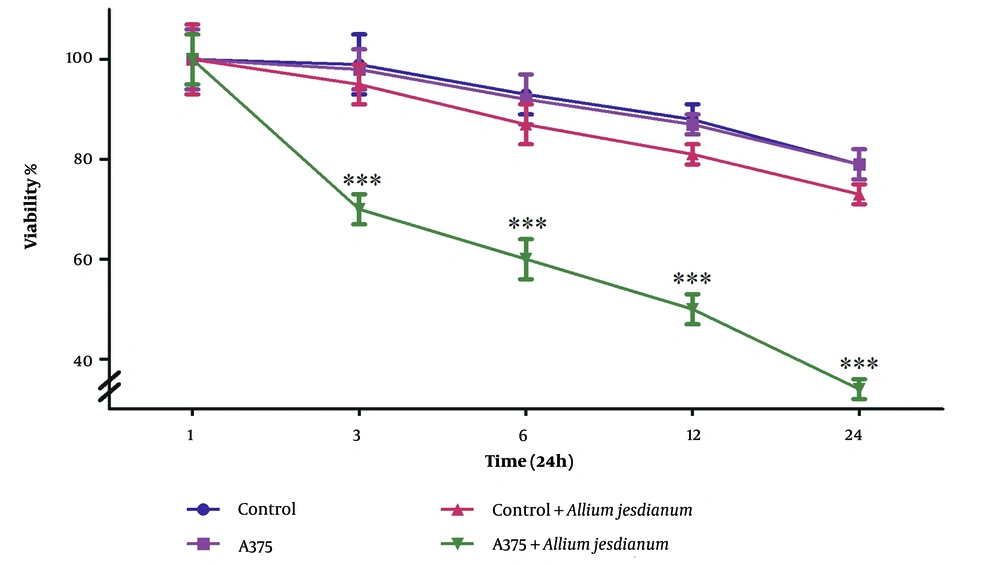
As shown in Figure 2, the A375 cell line treated with the extract exhibited a significant decrease in cell viability after 24 hours (P < 0.001). Conversely, no significant decrease was observed in AGO-1522 at this dose and duration.
4.3. Lipid Peroxidation
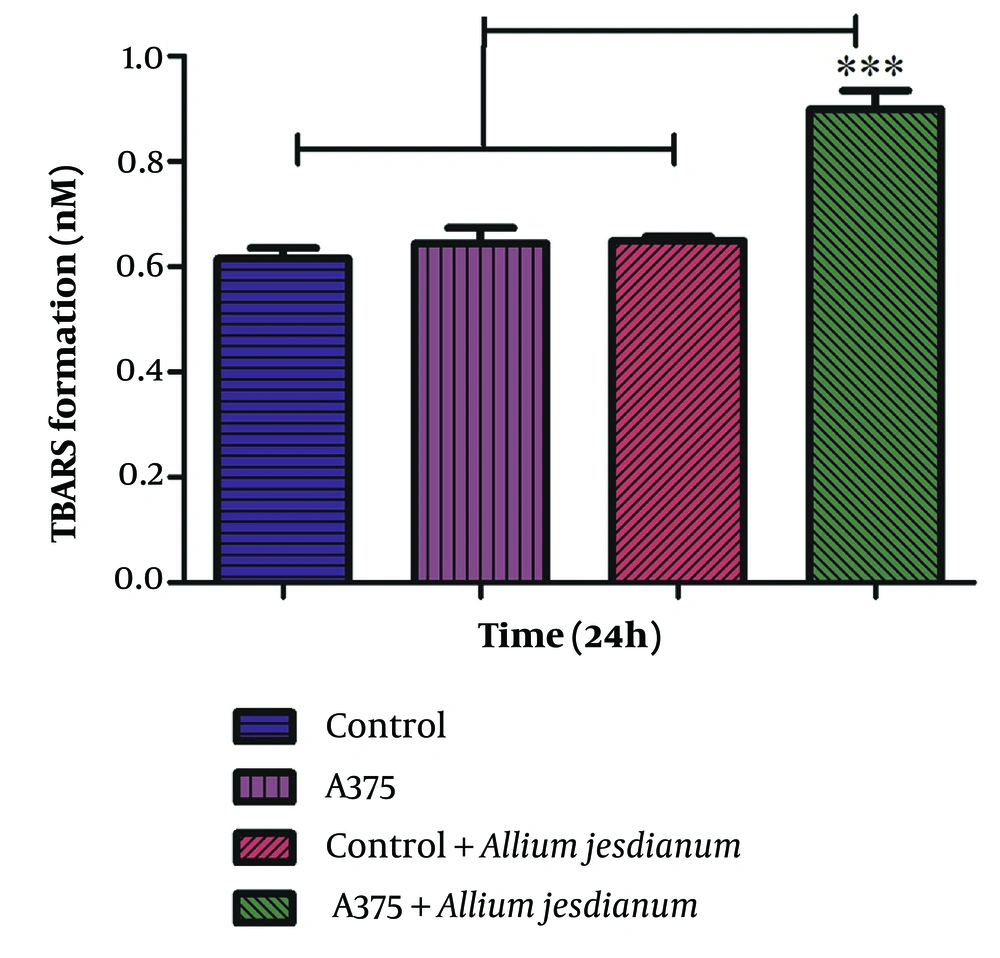
The addition of A. jesdianum extract to the A375 cell line substantially increased malondialdehyde (MDA) formation compared to the AGO-1522 cell line (P < 0.001) (Figure 3).
Lipid peroxidation activity of Allium jesdianum extract in human melanoma cell lines (A375) and normal fibroblast cell line (Control). Thiobarbituric acid-reactive substances (TBARS) formation was measured spectrophotometrically and expressed as μM concentrations. Values are indicated as mean ± standard deviation (SD) (n= 3). *** P < 0.001, a significant difference compared to control cells
4.4. Reactive Oxygen Species
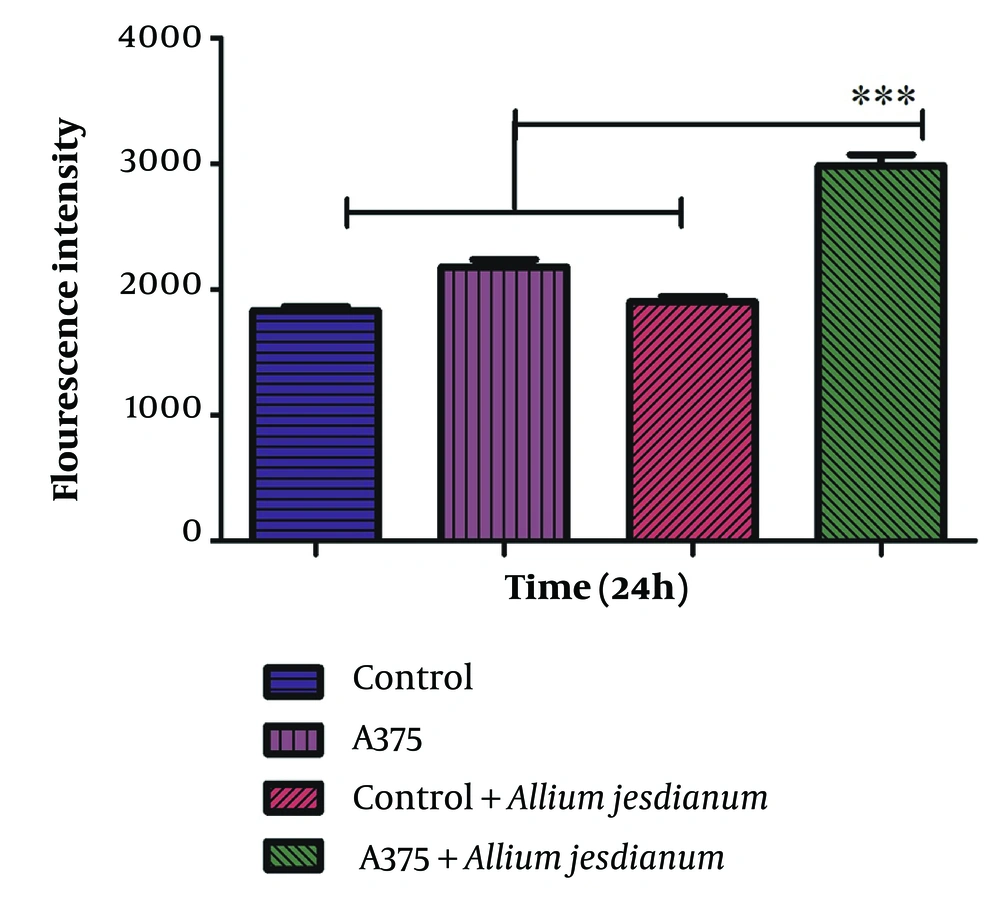
Figure 4 depicts a significant increase in the production of ROS in cancer cell lines when A. jesdianum was administered at a concentration of 0.1 mg/ml (P < 0.001). Conversely, no substantial rise in ROS formation was observed in normal fibroblast cells.
Effect of Allium jesdianum extract on the formation of reactive oxygen species using human melanoma cell lines (A375) and normal fibroblast cell line (Control). Reactive oxygen species were determined spectrofluorometrically by the measurement of highly fluorescent dichlorofluorescein (DCF). Values are indicated as mean ± standard deviation (SD) (n = 3). *** P < 0.001, a significant difference compared to control cells
4.5. Determination of Cytochrome c Release
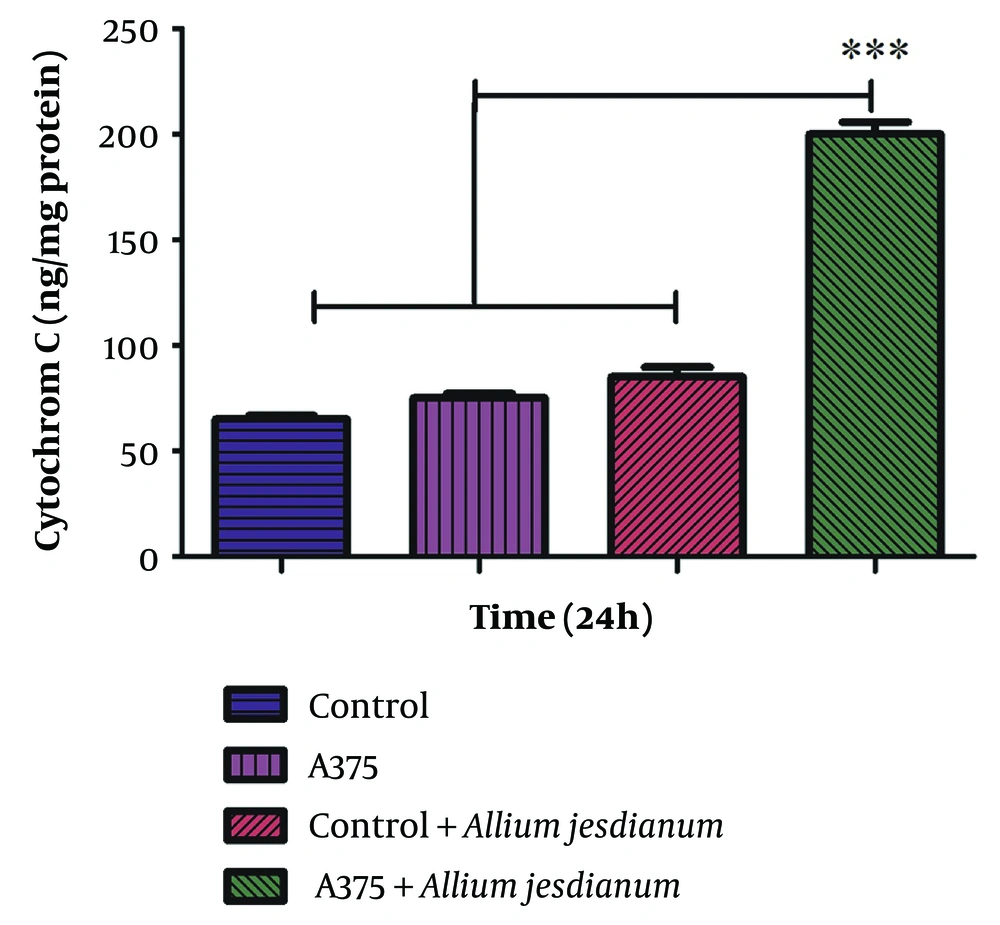
The administration of the A. jesdianum extract to the A375 cell line resulted in the release of CYT-C, indicating a decline in mitochondrial membrane potential and disruption of mitochondrial outer membrane integrity. However, no remarkable release of CYT-C was observed in the AGO-1522 cell line (Figure 5).
Effect of Allium jesdianum extract on the cytochrome c release using human melanoma cell line (A375) and normal fibroblast cell line (Control). Cytochrome c released was assayed spectrophotometrically (450 nm) by the enzyme-linked immunosorbent assay (ELISA) kit. Values are indicated as mean ± standard deviation (SD) (n = 3). *** P < 0.001, a significant difference compared to control cells
5. Discussion
Recent evidence suggests that Allium plants can reduce the risk of cancer by inhibiting carcinogen activation, promoting detoxification, and exhibiting antitumor properties and culinary uses in the region (22, 23). A specific species known as A. jesdianum, native to Iran’s Zagros Mountains, is utilized for both medicinal and culinary purposes in the region (24). The MTT study revealed that A. jesdianum had a cytotoxic impact on the melanoma cell line, leading to increased TBARS and ROS formation. Additionally, the release of CYT-C, indicating mitochondrial membrane potential collapse and depolarization, was observed in malignant melanoma cells.
The present study explored the potential anti-cancer effects of the total hydroalcoholic extract derived from A. jesdianum on melanoma cells. Extracts can contain multiple ingredients that target various pathways involved in cell death, such as apoptosis and cell cycle arrest, making them advantageous for treating complex diseases, such as cancer. For instance, several plant bioactive compounds, including polyphenols, flavonoids, and alkaloids, exhibit anti-carcinogenic properties (25, 26). Furthermore, considering the endangered status of A. jesdianum, utilizing a hydroalcoholic extract is a more economical option as it requires less plant material for extraction (27).
The present study provided novel findings indicating that the extract obtained from A. jesdianum possesses the ability to suppress the proliferation of human malignant melanoma cells. To achieve this, the present study examined the in vitro cytotoxic effects of the A. jesdianum extract against the A375 and AGO-1522 cell lines. The current study outcomes demonstrated a concentration- and time-dependent decrease in cell viability following treatment with the hydroalcoholic extract of A. jesdianum. Based on the findings of the present study, the IC50 of A. jesdianum extract for the tested cell lines was determined to be 0.1 mg/mL, exhibiting a significant suppressive effect on cell growth.
The results of the present study align with previous in vitro studies, indicating that steroidal glycosides from A. jesdianum bulbs exhibit cytostatic and cytotoxic activities against various malignant tumor cells (28). Furthermore, the results of the current study showed that the AGO-1522 cell line was not affected by A. jesdianum, suggesting the potential specificity of the extract toward cancer cells without harming healthy cells, such as fibroblasts. This significant finding suggests the safe potential usage of A. jesdianum extract in patients with malignant melanoma. Future studies should explore the anticancer effects of various fractions derived from this plant extract on melanoma, aiming to identify specific constituents within the extract that could serve as promising candidates for the development of a novel anticancer drug.
Another significant finding of this study was the notable induction of ROS formation in human melanoma cell lines when treated with A. jesdianum extract at a concentration of 0.1 mg/ml (P < 0.001). Importantly, normal fibroblast cells did not exhibit a significant increase in ROS formation. This finding holds particular relevance as conventional treatments, such as chemotherapy and radiation therapy, which elevate intracellular ROS levels in target cancer cells, can also affect surrounding normal cells, leading to deoxyribonucleic acid (DNA) damage and other biomolecular disruptions (29, 30).
The study also observed that treating the human melanoma cell line with A. jesdianum led to an increase in the production of MDA, a biomarker associated with oxidative stress and cell damage. Additionally, the treatment resulted in the release of CYT-C, which is associated with mitochondrial membrane potential collapse, depolarization, and apoptosis in malignant melanoma cells. The aforementioned findings suggest that the therapeutic mechanism of action of the A. jesdianum compound in cancer cells might involve MDA production and CYT-C release. This finding aligns with the findings of previous research on the Allium genus, as demonstrated by a study on garlic extract-induced cytotoxicity and apoptosis in the HL-60 model (human leukemia cells). This involved phosphatidylserine externalization, apoptosis activation, DNA fragmentation, and peroxidation (31).
Phytochemical analysis of various members of the Allium genus has revealed that these plants contain a wide array of secondary metabolites with pharmacological properties. These compounds, including S-allyl mercapto cysteine, quercetin, flavonoids, and ajoene, have demonstrated potential anticancer effects. To date, 16 species of Allium have been studied for their ability to inhibit cancer growth through diverse mechanisms, such as cell cycle arrest, inhibition of signaling pathways, induction of apoptosis, and antioxidant activity (32).
Polyphenol compounds, specifically flavonoids, have demonstrated promising therapeutic potential in various types of cancer (33). Previous studies have reported the anticancer activity of the A. jesdianum extract against human colon, cervical, myelogenous leukemia, and thyroid cancer cells by inducing oxidative stress and activating necroptosis signaling pathways (13-15). In the current study, the hydro-alcoholic extract of A. jesdianum was observed to contain 157.6 mg of phenols (gallic acid equivalent) and 114.7 mg of flavonoids (quercetin equivalent) per gram of dry extract. Furthermore, the extract induced apoptosis in malignant melanoma cells by triggering the release of CYT-C, which is related to the collapse of mitochondrial membrane potential and depolarization of the mitochondrial membrane. The aforementioned findings suggest that the observed effects might be attributed to the presence of these bioactive compounds that promote apoptosis (34).
5.1. Conclusions
The present study demonstrates that A. jesdianum exhibits cytotoxic effects on human malignant melanoma cells, potentially through the induction of oxidative stress and cancer cell apoptosis. These effects could be attributed to the presence of natural phytochemicals, such as organosulfur and flavonoids, in A. jesdianum extract, making it a promising candidate for cancer treatment. It is recommended to identify and isolate plant-derived anticancer agents in future studies and investigate the effect of these substances on other melanoma-derived cell lines and in vivo.