Abstract
Background:
Research shows that α-Pinene interacts with the opioidergic system.Objectives:
This study aims to examine the toxicity and the effects of α-Pinene on morphine tolerance and dependence in mice.Methods:
Guidelines No. 423 and No. 407 were used to investigate acute and sub-chronic toxicity, respectively. For sub-chronic toxicity analysis, the animals were sacrificed on day 28, and blood and tissue samples were collected. After inducing morphine tolerance or dependence, in both phases, animals received i.p. vehicle, diazepam (5 mg/kg), and α-Pinene (3.125, 6.25, and 12.5 mg/kg). Withdrawal signs were recorded for 30 minutes.Results:
Only the acute dose of α-Pinene showed mortality in animals, but mild lesions were seen in the brain, liver, and kidneys in the mice receiving its subchronic dose. Moreover, ALT, AST, ALP, and TG levels increased (P < 0.05) in female mice. Besides, 6.25 and 12.5 mg/kg (P < 0.001) of α-Pinene and only its high dose (12.5 mg/kg) (P < 0.001) reduced the number of jumps in the tolerance and dependence phases, respectively. Diarrhea (P < 0.001), writhing (P < 0.001), rearing, and climbing (P < 0.05 and P < 0.001, respectively) behaviors decreased in the tolerance phase, and grooming, climbing, and teeth chattering declined in the dependence phase (P < 0.001).Conclusions:
The LD50 of α-Pinene was lower than 2000 mg/kg, but its subchronic dose caused mild tissue toxicities and biochemical changes. Moreover, α-Pinene decreased morphine tolerance and dependence and possibly was useful for the treatment of opioid dependence after complimentary trials.Keywords
1. Background
Dependence on opioids (e.g., morphine) is a serious disease that causes enormous health problems and economic losses for individuals and societies (1). Morphine, which is used to relieve acute and chronic pain, promotes its analgesic effects through the modulation of μ opioid receptors (2). However, the continuous use of morphine leads to euphoria, respiratory depression, constipation, miosis, tolerance, and dependence, limiting its clinical use (3). In the tolerance and dependence phases, unique changes happen during administration and after drug cessation, respectively (4). Tolerance gradually reduces the effectiveness of morphine, so higher doses are required to relieve pain. Morphine dependence is associated with ailments such as anxiety, abdominal pain, diarrhea, etc. Earlier studies have shown that the continuous use of morphine affects the mesolimbic dopaminergic system in the ventral tegmental area (VTA) and leads to the development of tolerance and dependence (5, 6). In this regard, studies have shown that several neural systems, such as the GABAergic and opioidergic systems, modulate the activity of the dopaminergic system, contributing to opioid tolerance and dependence (5, 6).
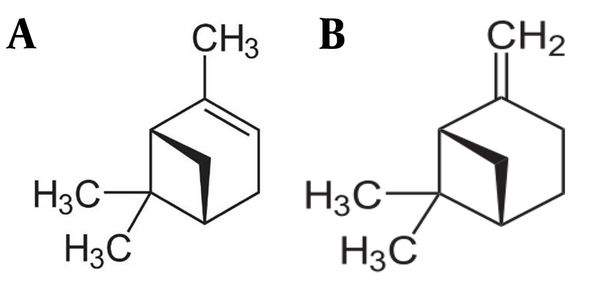
α-Pinene (Alpha-pinene) (Figure 1A) is a bicyclic monoterpene existing in many aromatic plants, especially pine oil. This compound is a colorless liquid with low water solubility, high oil content, and high ethanol solubility and is used in the flavor, fragrance, paper, pharmaceutical, and paint industries (7). Various pharmacological properties, including antiseizure, antinociceptive, anti-inflammatory, antioxidant, and cytoprotective effects, have been attributed to α-Pinene (8-11). Moreover, a study showed that α-Pinene, with GABAergic activity, induced hypnotic and anxiolytic-like effects (12). In contrast, previous studies demonstrate that β-Pinene (as well as other Pinene structural isomers) (Figure 1B) exhibits antispasmodic, anti-inflammatory, hypotensive, antifungal, antihypertensive, antimicrobial, anti-depressant, and sedative activities (13-15). Moreover, a study demonstrated that β-Pinene (50 - 100 mg /kg) could alleviate some morphine withdrawal signs (16).
Structure of (-)-α-Pinene(A), and β-Pinene (B)
2. Objectives
No study has been conducted on the effects of α-Pinene on morphine tolerance and dependence. However, the clinical use of the appropriate doses of α-Pinene requires knowing its toxicity effects. Therefore, we investigated the toxicity and biological effects of α-Pinene on morphine tolerance and dependence in mice.
3. Methods
3.1. Animals
Six-week-old NMRI male or female mice (22 - 32 g) were obtained from Pasture Institute (Tehran, Iran). The animals were maintained in 12 h light/dark cycles at 21 - 23°C with free access to water and food.
3.2. Chemicals
The chemicals used were as follows: (-)-α-Pinene and naloxone hydrochloride (Sigma Co, USA), and morphine sulfate and diazepam hydrochloride (Darou Pakhsh Co, Iran). In this study, agents were dissolved in Tween 80 (2%) (Merck, Germany) and administrated orally (p.o.) or intraperitoneally (i.p.) in a volume of 0.1 mL/10 g. Control groups received normal saline with 2% Tween 80 as a vehicle (10 mL/kg).
3.3. Toxicity
Acute toxicity was conducted based on guideline No. 423 (17). A single p.o. dose of 2000 mg/kg of α-Pinene was administered. Control groups received vehicles. Mortality and food or water intake, etc., were monitored in mice. Mortality was investigated for 30 minutes, then continuously for 4 - 24 hours, and then twice daily for 14 days after the administration of α-Pinene (18).
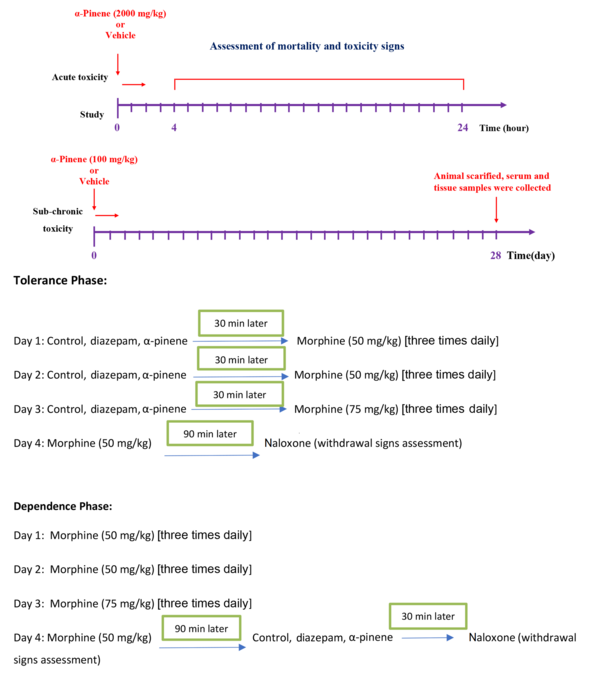
The toxicity of the subchronic p.o. dose of α-Pinene was assessed as recommended in the OECD guideline No. 407 (18). Mice received α-Pinene at a dose of 100 mg/kg for 28 days and then were allocated into two groups, including the vehicle- and α-Pinene-treated group (n = 6). On the last day, the animals were sacrificed, and sera and tissue sections of the brain, kidney, liver, and small intestine were harvested (Figure 2).
The experimental design for analyzing the toxicity and beneficial effects of α-Pinene on morphine tolerance and dependence
For biochemical assessments, the animal’s heart blood was collected into dry tubes and then centrifuged at 3000 r.p.m. (4°C) for 15 minutes. The isolated sera were stored at 20°C for biochemical assessments [blood glucose, blood urea nitrogen (BUN), ALT (alanine aminotransferase), AST (aspartate aminotransferase), ALP (alkaline phosphatase), total cholesterol, triglycerides (TG), and Cr (creatinine)] using specific kits manufactured by Pars Azmun Co. (Tehran, Iran).
For histopathological assessments, the organs were fixed in formalin (10%), then dehydrated, embedded in paraffin wax, sectioned at a 5-μm thickness, and finally stained with hematoxylin/eosin (H&E). A light microscope (Olympus, Japan) was utilized to evaluate histopathological changes.
3.4. Induction of Tolerance and Dependence
In order to induce morphine tolerance or dependence, this compound was administered i.p. three times daily at 8 a.m. (50 mg/kg), 12 p.m. (50 mg/kg), and 4 p.m. for 3 continuous days, followed by the administration of a single dose (50 mg/kg) on day 4 before administration of naloxone (19, 20).
3.5. Grouping and Experimental Design
3.5.1. Tolerance Phase
Thirty mice received either the vehicle (n = 6, negative control), diazepam (5 mg/kg, n = 6, positive control), and α-Pinene at the doses of 3.125, 6.25, and 12.5 mg/kg (treatment groups, n = 6 in each group), accompanied by morphine administration three times per day, but not on day 4. On day 4, 2 hours after the final dose of morphine (50 mg/kg), the animals received naloxone (2 mg/kg), and withdrawal signs were recorded.
3.5.2. Dependence Phase
In a separate experiment, after the 3-day schedule of morphine administration and 30 min after the final dose of morphine (50 mg/kg) on day fourth, 30 animals (n = 6) received the same treatments and α-Pinene doses used in the tolerance phase. Next, 1.5 hours later, the animals received naloxone, and withdrawal signs were recorded (Figure 2). The study design and the doses of the drugs used were chosen based on previous studies (12, 19-22).
3.6. Monitoring of Morphine Withdrawal Signs
The animals were placed on filter paper in a glass beaker (15 × 50 cm) and investigated for 30 minutes by a digital camera. Withdrawal signs were recorded by counting jumps. The severity of the signs was scored for teeth chattering, diarrhea, climbing, grooming, diarrhea, rearing, and writhing. The items were scored in a score range of 1 - 3 as follows: Mild (1), moderate (2), and (3) severe (23).
3.7. Statistical Analysis
Data with non-normal distribution were expressed as medians with 25th-75th percentiles and analyzed by the Kruskal-Wallis test. A post-hoc test using Dunn’s test with Bonferroni correction was used for non-parametric pairwise multiple comparisons between independent groups. In all analyses, P < 0.05 was considered statistically significant. SPSS software v. 25 and GraphPad Prism 9.1 were used for data analysis and preparation of graphs, respectively.
4. Results
4.1. Biochemical Changes
As shown in Table 1, α-Pinene (100 mg/kg) significantly increased liver function parameters, including ALT (P = 0.0373), AST (P = 0.0156), and ALP (P = 0.0370), compared to control groups in female mice. Moreover, this dose of α-Pinene significantly increased serum TG (P = 0.0217) but not total cholesterol (P > 0.05). In addition, kidney function parameters (BUN and Cr) and blood glucose were not significantly changed compared to control groups (P > 0.05). In contrast, none of these serum parameters were influenced by α-Pinene (100 mg/kg) in male mice.
| Parameters | Mice | |||
|---|---|---|---|---|
| Female | Male | |||
| Test | Control | Test | Control | |
| Blood glucose (mg/dL) | 198.83 ± 5.81 | 192.83 ± 5.14 | 203.00 ± 6.88 | 192.83 ± 5.1 |
| BUN (mg/dL) | 56.67 ± 3.32 | 59.00 ±2.80 | 62.67 ± 2.74 | 59.00 ±2.80 |
| Cr (mg/dL) | 0.39 ± 0.01 | 0.41 ± 0.01 | 0.40 ± 0.01 | 0.41 ± 0.01 |
| Total cholesterol (mg/dL) | 142.17 ± 9.49 | 118.50 ± 5.64 | 126.33 ± 10.12 | 118.50 ± 5.64 |
| TG (mg/dL) | 212.00 ± 5.70 c, P = 0.0217 | 186.33 ± 7.16 | 207.50 ± 4.87 | 186.33 ± 7.16 |
| AST IU/l | 167.33 ± 7.27 c, P = 0.0156 | 138.50 ± 4.78 | 160.83 ± 6.79 | 138.50 ± 4.78 |
| ALT IU/l | 104.33 ± 4.54 c, P = 0.0373 | 83.67 ± 6.25 | 100.00 ±4.98 | 83.67 ± 6.25 |
| ALP IU/l | 176.00 ± 10.26 c, P = 0.0370 | 140.33 ± 6.82 | 165.67 ± 9.97 | 140.33 ± 6.82 |
4.2. Histopathological Parameters
According to the results, the lethal dose of 50 % (LD50) of α-Pinene was lower than 2000 mg/kg. As shown in Figure 3, vasogenic edema (E) was seen in some regions of the brain in the control (Part A) group and experimental (Part B) groups in the mice of both sexes, but meninges and other cells appeared normal. As shown in Figure 3, cell swelling was seen in the control group in both sexes (Panel A). Moreover, there were signs of inflammation and fibrosis in some regions of the liver in α-Pinene-treated female mice (Panel B).
Histological assessment of the brain in the control (vehicle) (A) and experiment (B) groups [arrows show vasogenic edema]; liver in the control (C) and treatment (D) groups [arrows show inflammatory infiltrates]; kidney in the control (E) and treatment (F) groups [arrows show diffuse hemorrhage]. In the treatment group, subacute glomerulonephritis is seen (yellow arrow)], and the small intestine appeared normal in the control (G) and treatment (H) groups
![Histological assessment of the brain in the control (vehicle) (A) and experiment (B) groups [arrows show vasogenic edema]; liver in the control (C) and treatment (D) groups [arrows show inflammatory infiltrates]; kidney in the control (E) and treatment (F) groups [arrows show diffuse hemorrhage]. In the treatment group, subacute glomerulonephritis is seen (yellow arrow)], and the small intestine appeared normal in the control (G) and treatment (H) groups Histological assessment of the brain in the control (vehicle) (A) and experiment (B) groups [arrows show vasogenic edema]; liver in the control (C) and treatment (D) groups [arrows show inflammatory infiltrates]; kidney in the control (E) and treatment (F) groups [arrows show diffuse hemorrhage]. In the treatment group, subacute glomerulonephritis is seen (yellow arrow)], and the small intestine appeared normal in the control (G) and treatment (H) groups](https://services.brieflands.com/cdn/serve/31648/e7b09b360cae8f9301906416c99075cc1231c312/jjnpp-18-4-141534-g001-preview.png)
As illustrated in Figure 3, mild kidney hemorrhage was observed in the control (Panel A) and experimental (Panel B) groups in both sexes. There were no pathologic changes in the villi of the small intestine in the control (Panel A) and α-Pinene-treated (Panel B) groups. Overall, histopathological evaluation in the control and α-Pinene-treated group showed normal structures without any gross pathological lesions in different organs in both sexes.
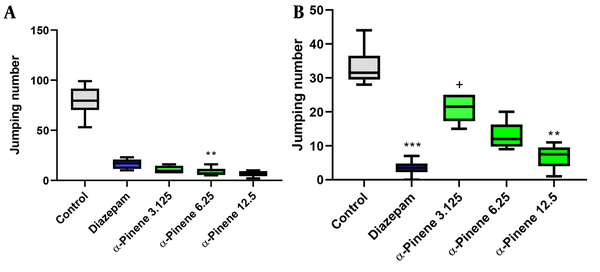
4.3. The Effect of α-Pinene on Morphine Withdrawal Signs During the Tolerance Phase in Male Mice
As shown in Figure 4A, 6.25 and 12.5 mg/kg doses of α-Pinene reduced the number of jumps compared to the control group (P = 0.002 and P = 0.001, respectively). In addition, diazepam (5mg/kg) could not decrease the number of jumps (P > 0.05). Nevertheless, insignificant differences were observed between animals treated with α-Pinene and diazepam (as the positive control) (P > 0.05).
Effects of different doses of α-Pinene (3.125, 6.25, and 12.5 mg/kg) and diazepam (5 mg/kg) on the number of jumpings during the tolerance (Panel A) and development (Panel B) phases. The data were expressed as the median (min to max) and analyzed using the Kruskal-Wallis test and post-hoc Bonferroni correction (n = 6 mice per group). SPSS 25 and GraphPad Prism 9.1 were used for data analysis and drawing the graphs, respectively. ** shows significant differences at P < 0.01 compared to the control group. *** shows significant differences at P < 0.001 compared to the control group. + shows significant differences at P < 0.05 compared to the diazepam group
As shown in Table 2, all doses of α-Pinene reduced diarrhea (P = 0.002 for all doses), writhing (P = 0.001 for all doses), and climbing (P = 0.049, P = 0.049, and P = 0.002, respectively). In this phase, 6.25 and 12.5 mg/kg doses of α-Pinene decreased rearing (P = 0.014, for both doses). The administration of α-Pinene did not cause significant changes in grooming and teeth chattering (P > 0.05). Moreover, diazepam (5 mg/kg) reduced diarrhea (P = 0.002), writhing (P = 0.031), rearing (P = 0.001), climbing (P = 0.002), and grooming (P = 0.009) during the tolerance phase.
Effects of Different Doses of α-Pinene and Diazepam During the Tolerance and Development Phases a, b, c, d
| Treatments | Diarrhea | Writhing | Rearing | Climbing | Grooming | Teeth Chattering |
|---|---|---|---|---|---|---|
| Tolerance | ||||||
| Vehicle | (2.0 - 3.0) 3 | (2.5 - 3.0) 3 | (2.0 - 3.0) 2.5 | (2.0 - 2.25) 2 | (1.0 - 2.25) 2 | (1.0 - 3.0) 1 |
| Diazepam 5 mg/kg | (0.75 - 1.25) 1 ** | (0.0 - 1.0) 0* | (0.0 - 1.0) 0.5 ** | (0.0 - 1.0) 0.5** | (0.0 - 1.0) 0.5* | (0.75 - 1.0) 1 |
| α-Pinene 3.125 mg/kg | (1.0 - 1.0) 1 ** | (0.0 - 0.0) 0 ** | (1.0 - 2.0) 1 | (0.75 - 1.0) 1* | (1.0 - 2.0) 1 | (1.0 - 1.0) 1 |
| α-Pinene 6.25 mg/kg | (1.0 - 1.0) 1** | (0.0 - 0.0) 0** | (1.0 - 1.0) 1* | (0.75 - 1.0) 1* | (0.75 - 1.0) 1 | (0.75 - 1.0) 1 |
| α-Pinene 12.5 mg/kg | (1.0 - 1.0) 1 ** | (0.0 - 0.0) 0 ** | (1.0 - 1.0) 1* | (0.0 - 1.0) 0.5 ** | (0.75 - 1.0) 1 | (0.75 - 1.0) 1 |
| Dependence | ||||||
| Vehicle | (1.75 - 2.25) 2 | (0.0 - 2.25) 0 | (1.0 - 2.0) 2 | (1.0 - 2.0) 2 | (1.75 - 2.0) 2 | (1.75 - 2.0) 2 |
| Diazepam 5 mg/kg | (0.0 - 1.0) 0** | (0.0 - 0.0) 0 | (0.0 - 1.0) 0** | (0.0 - 2.0) 1.5 | (0.0 - 2.0) 2 | (1.0 - 2.25) 2 |
| α-Pinene 3.125 mg/kg | (0.0 - 1.0) 1 | (0.0 - 0.0) 0 | (1.0 - 1.25) 1 | (0.0 - 1.0) 0.5 | (0.75 - 1.0) 1 | (0.0 - 0.0) 0 ** ++ |
| α-Pinene 6.25 mg/kg | (0.0 - 1.0) 1 | (0.0 - 0.0) 0 | (1.0 - 1.0) 1 | (0.0 - 1.0) 1 | (1.0 - 1.0) 1 | (0.0 - 0.0) 0 ** ++ |
| α-Pinene 12.5 mg/kg | (0.0 - 1.0) 1 | (0.0 - 0.0) 0 | (0.0 - 1.0) 1 | (0.0 - 0.0) 0 ** | (0.0 - 0.0) 0 ** + | (0.0 - 0.0) 0 ** ++ |
4.4. The Effect of α-Pinene on Morphine Withdrawal Signs During the Dependence Phase in Male Mice
As illustrated in Figure 3B, a high dose (12.5 mg/kg) of α-Pinene (P = 0.003) and diazepam (P = 0.001) decreased the number of jumps during the dependence phase. Diazepam decreased the number of jumping episodes significantly better than low-dose α-Pinene (3.125 mg/kg) (P = 0.012).
As shown in Table 2, all doses of α-Pinene decreased teeth chattering (P = 0.007 for all doses). The administration of α-Pinene did not have effects on diarrhea and writhing (P > 0.05). In addition, only high-dose α-Pinene decreased climbing and grooming behaviors (P = 0.003 and P = 0.001, respectively).
In the dependence phase, diazepam (5 mg/kg) reduced diarrhea and rearing (P = 0.003 and P = 0.002, respectively). Moreover, all doses of α-Pinene performed better than diazepam in decreasing teeth chattering (P = 0.001), and high-dose α-Pinene performed better than diazepam in decreasing grooming (P = 0.001).
5. Discussion
The present study aimed to examine the toxicity and biological effects of α-Pinene on morphine dependence and tolerance in a mouse model. Phytotherapeutic products, such as active compounds, are used for their healthcare effects, particularly in developing countries, and are usually derived from natural sources. However, phytotherapeutic products are not completely safe, and thus, it is necessary to investigate their therapeutic effectiveness and biosafety (23, 24). In vivo toxicity analysis is generally performed to evaluate the acute toxicity of medicinal plants. A toxic agent may show good pharmacological effects at lower non-toxic doses, suggesting their potential to be used to produce advanced pharmacological products (25). Acute toxicity analysis is used to obtain a suitable dose for chronic toxicity testing.
According to our results, the administration of α-Pinene caused vasogenic edema in some regions of the brain, as well as inflammation and fibrosis in the liver and hemorrhage in the kidneys. Moreover, ALT, AST, ALP, and TG were significantly increased only in female mice. These results were also observed in animals in the control groups, indicating that α-Pinene did not have sub-chronic toxicity or adverse effects on histopathological parameters. Similar to our findings, a study demonstrated that monoterpenes (e.g., α-Pinene) reduced the viability of Vero (monkey kidney) cells (26).
Our study, for the first time, showed that α-Pinene alleviated morphine withdrawal signs during the tolerance and development phases. In line with our results, a study demonstrated that only high-dose (200 mg/kg) β-pinene could attenuate jumping, rearing, and diarrhea in morphine-dependent mice (12). However, the number of withdrawal signs assessed in our study was much higher than in the recent study, and also, we used low doses of α-pinene (3.125 - 12.5 mg/kg) compared to the doses of β-pinene (50 - 200 mg/kg) mentioned above. Unlike the recent study, we also explored the histotoxicity effects of α-pinene. In line with our findings, phytochemicals have been noted to exhibit a variety of toxicological and biological activities (14). Hence, the novelty of our study lies not only in its design but also in the results obtained.
Another study showed that α-pinene, with GABAergic activity, induced hypnotic- and anxiolytic-like effects, which is consistent with its specific binding sites on GABAA benzodiazepine (BZD) receptors (12). With this in mind, previous studies have demonstrated that the GABAergic system performs a pivotal function in opioid addiction. Moreover, studies have shown that positive modulators on the benzodiazepine site in the GABAA receptors (e.g., diazepam) and selective GABAA receptor agonists (e.g., muscimol) pacify morphine withdrawal signs (6, 19, 20, 27).
It was discussed that opioid receptors (e.g., μ receptors) are important in the treatment of pain (28). In this regard, a study showed that α-Pinene decreased pupal pain by acting on GABAA and μ opioid receptors (29). Studies have also reported that the administration of naloxone blocks the analgesic effects of other monoterpenes (e.g., p-cymene and/or citronellal) (30, 31). Apparently, α-pinene can activate endogenous opioid neurotransmitters in the brain and may suppress downstream nociceptive pathways. In summary, the administration of α-pinene may decrease withdrawal signs, at least in part via the modulation of GABAergic or opioidergic systems.
Inflammation plays a key role in the development of morphine withdrawal signs. In this regard, a study showed that α-pinene decreased the production of inflammatory mediators (8, 26). The results of another study showed that α-pinene decreased the expression of cyclooxygenase-2 (COX-2), which is involved in the pathogenesis of inflammatory pain (32). Overall, α-pinene can decrease inflammation, which can partly explain its role in mitigating morphine tolerance and dependence. Another mechanism that can be involved in the role of α-pinene in alleviating morphine withdrawal symptoms is through antioxidant pathways. It has been reported that α-pinene plays a pivotal role in decreasing apoptosis and protecting the nervous system through boosting antioxidant pathways (9). So, α-pinene may decrease morphine tolerance or dependence by inducing antioxidant routes.
5.1. Conclusions
Our results demonstrated that the LD50 of α-pinene was lower than 2000 mg/kg, but the subchronic dose of this compound caused mild tissue toxicities. Moreover, α-pinene decreased the symptoms of morphine tolerance and dependence at lower doses compared to β-Pinene. A limitation of this study was that it was conducted on mice, and thus, the results obtained could not be generalized to humans. Hence, α-pinene can be suggested as an agent for the management of morphine dependence and tolerance.
References
-
1.
Sheikh-Sarmast HA, Abbasi-Maleki S. The Effects of Cymbopogon citratus (lemon grass) on Morphine Withdrawal Signs in Male Mice. Herb Med J. 2018:8-13.
-
2.
Basiri F, Rad A, Mahdian D, Molavi M, Amin B. Effects of glucosamine against morphine-induced antinociceptive tolerance and dependence in mice. J Biomed Sci. 2019;26(1):21. [PubMed ID: 30782159]. [PubMed Central ID: PMC6380027]. https://doi.org/10.1186/s12929-019-0513-1.
-
3.
Safakhah HA, Damghanian F, Bandegi AR, Miladi-Gorji H. Effect of crocin on morphine tolerance and serum BDNF levels in a rat model of neuropathic pain. Pharmacol Rep. 2020;72(2):305-13. [PubMed ID: 32112363]. https://doi.org/10.1007/s43440-020-00071-9.
-
4.
Volkow ND, Michaelides M, Baler R. The Neuroscience of Drug Reward and Addiction. Physiol Rev. 2019;99(4):2115-40. [PubMed ID: 31507244]. [PubMed Central ID: PMC6890985]. https://doi.org/10.1152/physrev.00014.2018.
-
5.
Alipour P, Khodayar MJ, Mansouri MT, Ghorbanzadeh B. Investigation on the Effect of Ketotifen Upon Morphine Tolerance and Dependence in Mice. Jundishapur J Nat Pharm Prod. 2018;In Press(In Press). https://doi.org/10.5812/jjnpp.16303.
-
6.
Hosseinzadeh Sahafi O, Sardari M, Alijanpour S, Rezayof A. Shared Mechanisms of GABAergic and Opioidergic Transmission Regulate Corticolimbic Reward Systems and Cognitive Aspects of Motivational Behaviors. Brain Sci. 2023;13(5). [PubMed ID: 37239287]. [PubMed Central ID: PMC10216078]. https://doi.org/10.3390/brainsci13050815.
-
7.
Wu CS, Chen YJ, Chen JJ, Shieh JJ, Huang CH, Lin PS, et al. Terpinen-4-ol Induces Apoptosis in Human Nonsmall Cell Lung Cancer In Vitro and In Vivo. Evid Based Complement Alternat Med. 2012;2012:818261. [PubMed ID: 21760828]. [PubMed Central ID: PMC3133878]. https://doi.org/10.1155/2012/818261.
-
8.
Kim DS, Lee HJ, Jeon YD, Han YH, Kee JY, Kim HJ, et al. Alpha-Pinene Exhibits Anti-Inflammatory Activity Through the Suppression of MAPKs and the NF-kappaB Pathway in Mouse Peritoneal Macrophages. Am J Chin Med. 2015;43(4):731-42. [PubMed ID: 26119957]. https://doi.org/10.1142/S0192415X15500457.
-
9.
Porres-Martinez M, Gonzalez-Burgos E, Carretero ME, Gomez-Serranillos MP. Major selected monoterpenes alpha-pinene and 1,8-cineole found in Salvia lavandulifolia (Spanish sage) essential oil as regulators of cellular redox balance. Pharm Biol. 2015;53(6):921-9. [PubMed ID: 25474583]. https://doi.org/10.3109/13880209.2014.950672.
-
10.
Turkez H, Aydin E. In vitro assessment of cytogenetic and oxidative effects of alpha-pinene. Toxicol Ind Health. 2016;32(1):168-76. [PubMed ID: 24081629]. https://doi.org/10.1177/0748233713498456.
-
11.
Bouzenna H, Hfaiedh N, Giroux-Metges MA, Elfeki A, Talarmin H. Potential protective effects of alpha-pinene against cytotoxicity caused by aspirin in the IEC-6 cells. Biomed Pharmacother. 2017;93:961-8. [PubMed ID: 28724214]. https://doi.org/10.1016/j.biopha.2017.06.031.
-
12.
Yang H, Woo J, Pae AN, Um MY, Cho NC, Park KD, et al. alpha-Pinene, a Major Constituent of Pine Tree Oils, Enhances Non-Rapid Eye Movement Sleep in Mice through GABAA-benzodiazepine Receptors. Mol Pharmacol. 2016;90(5):530-9. [PubMed ID: 27573669]. https://doi.org/10.1124/mol.116.105080.
-
13.
Guzman-Gutierrez SL, Gomez-Cansino R, Garcia-Zebadua JC, Jimenez-Perez NC, Reyes-Chilpa R. Antidepressant activity of Litsea glaucescens essential oil: identification of beta-pinene and linalool as active principles. J Ethnopharmacol. 2012;143(2):673-9. [PubMed ID: 22867633]. https://doi.org/10.1016/j.jep.2012.07.026.
-
14.
Rivas da Silva AC, Lopes PM, Barros de Azevedo MM, Costa DC, Alviano CS, Alviano DS. Biological activities of alpha-pinene and beta-pinene enantiomers. Molecules. 2012;17(6):6305-16. [PubMed ID: 22634841]. [PubMed Central ID: PMC6268778]. https://doi.org/10.3390/molecules17066305.
-
15.
Park BB, An JY, Park SU. Recent studies on pinene and its biological and pharmacological activities. EXCLI journal. 2021;20:812-8.
-
16.
Zehtabi S, Ghafghazi S, Sheikholeslami MA, Parvardeh S. [Attenuating effect of β-pinene, a bicyclic monoterpene, on the acquisition and expression of morphine dependence in mice]. 8th basic and clinical neuroscience congress. 1398. Persian.
-
17.
Guideline PT. OECD guideline for the testing of chemicals. The Hershberger. 2001;601:858.
-
18.
Daneshbakhsh D, Asgarpanah J, Najafizadeh P, Rastegar T, Mousavi Z. [Safety Assessment of Mentha mozaffarianii Essential Oil: Acute and Repeated Toxicity Studies]. Iran J Med Sci. 2018;43(5):I479-86. Persian.
-
19.
Shirzadi D, Abbasi-Maleki S, Zanbouri A. Ethanolic extract of anise (Pimpinella anisum L.) attenuates morphine physical dependence in mice. J Herbmed Pharmacol. 2017;6(2):69-73.
-
20.
Hosseinzadeh H, Parvardeh S, Masoudi A, Moghimi M, Mahboobifard F. Attenuation of morphine tolerance and dependence by thymoquinone in mice. Avicenna J Phytomed. 2016;6(1):55-66. [PubMed ID: 27247922]. [PubMed Central ID: PMC4884218].
-
21.
Hosseinzadeh H, Ziaee T. [Effect of Nepeta glomerulosa Boiss. Aerial parts aqueous extract on morphine withdrawal syndrome in mice]. Iran J Pharmaceutic Sci. 2006. Persian.
-
22.
Hosseinzadeh H, Imenshahidi M, Hosseini M, Razavi BM. Effect of linalool on morphine tolerance and dependence in mice. Phytother Res. 2012;26(9):1399-404. [PubMed ID: 22318903]. https://doi.org/10.1002/ptr.3736.
-
23.
Vaghasiya YK, Shukla VJ, Chanda SV. Acute Oral Toxicity Study of Pluchea arguta Boiss Extract in Mice. J Pharmacol Toxicol. 2011;6(2):113-23. https://doi.org/10.3923/jpt.2011.113.123.
-
24.
Moreira DDL, Teixeira SS, Monteiro MHD, De-Oliveira ACA, Paumgartten FJ. Traditional use and safety of herbal medicines1. Rev Brasde Farmacogn. 2014;24(2):248-57. https://doi.org/10.1016/j.bjp.2014.03.006.
-
25.
Moshi MJ, van den Beukel CJ, Hamza OJ, Mbwambo ZH, Nondo RO, Masimba PJ, et al. Brine shrimp toxicity evaluation of some Tanzanian plants used traditionally for the treatment of fungal infections. Afr J Tradit Complement Altern Med. 2006;4(2):219-25. [PubMed ID: 20162095]. [PubMed Central ID: PMC2816448]. https://doi.org/10.4314/ajtcam.v4i2.31211.
-
26.
Silva SLD, Figueiredo PM, Yano T. Cytotoxic evaluation of essential oil from Zanthoxylum rhoifolium Lam. leaves. Acta Amazonica. 2007;37(2):281-6. https://doi.org/10.1590/s0044-59672007000200015.
-
27.
Tejwani GA, Sheu MJ, Sribanditmongkol P, Satyapriya A. Inhibition of morphine tolerance and dependence by diazepam and its relation to mu-opioid receptors in the rat brain and spinal cord. Brain Res. 1998;797(2):305-12. [PubMed ID: 9666154]. https://doi.org/10.1016/s0006-8993(98)00416-8.
-
28.
Al-Hasani R, Bruchas MR. Molecular mechanisms of opioid receptor-dependent signaling and behavior. Anesthesiology. 2011;115(6):1363-81. [PubMed ID: 22020140]. [PubMed Central ID: PMC3698859]. https://doi.org/10.1097/ALN.0b013e318238bba6.
-
29.
Rahbar I, Abbasnejad M, Haghani J, Raoof M, Kooshki R, Esmaeili-Mahani S. The effect of central administration of alpha-pinene on capsaicin-induced dental pulp nociception. Int Endod J. 2019;52(3):307-17. [PubMed ID: 30152861]. https://doi.org/10.1111/iej.13006.
-
30.
Santana MF, Quintans-Júnior LJ, Cavalcanti SC, Oliveira MG, Guimarães AG, Cunha ES, et al. p-Cymene reduces orofacial nociceptive response in mice. Rev Bras Farmacogn. 2011;21(6):1138-43. https://doi.org/10.1590/s0102-695x2011005000156.
-
31.
Quintans-Júnior LJ, Melo MS, De Sousa DP, Araujo AA, Onofre AC, Gelain DP, et al. Antinociceptive effects of citronellal in formalin-, capsaicin-, and glutamate-induced orofacial nociception in rodents and its action on nerve excitability. J Orofac Pain. 2010;24(3):305-12.
-
32.
Li XJ, Yang YJ, Li YS, Zhang WK, Tang HB. alpha-Pinene, linalool, and 1-octanol contribute to the topical anti-inflammatory and analgesic activities of frankincense by inhibiting COX-2. J Ethnopharmacol. 2016;179:22-6. [PubMed ID: 26721216]. https://doi.org/10.1016/j.jep.2015.12.039.
