1. Background
Cellular damage induced by oxidative stress is a hallmark of neurodegenerative disorders (1). Low concentrations of reactive oxygen species (ROS) are involved in biological processes. In contrast, excessive ROS production and inadequate antioxidant defenses lead to oxidative stress, disrupting various signaling pathways (2). Oxidative stress can lead to various molecular dysfunctions and neuronal apoptosis in the neurodegenerative system. Hence, scavenging free radicals using antioxidant agents is promising for enhancing neuronal antioxidant capacity (3).
Studies have demonstrated that phenolic compounds are natural antioxidants that disrupt cellular redox. Date palm (Phoenix dactylifera L.) pollen (DPP), widely used in traditional medicine, has been shown to contain a variety of biochemicals, including carbohydrates, amino acids, fatty acids, vitamins, dietary fiber, estrogenic constituents, hormones, and antioxidant agents (4, 5). Furthermore, DPP has been shown to possess antibiotic, anti-inflammatory, and antioxidant properties, potentially offering benefits for various human diseases (6).
One of the main challenges of neurodegeneration is the alteration of neuroplasticity features. Studies have shown that antidepressant drugs can influence neuroplasticity and may possess antioxidant properties. Fluvoxamine maleate (Flv) is a selective serotonin reuptake inhibitor (SSRI) used to treat obsessive-compulsive disorder (OCD), a neurodegenerative condition (7, 8). Different molecular pathways can regulate the antioxidant response elements (ARE) pathway. Nuclear factor erythroid 2-related factor 2 (NRF-2; gene name of NFE2L2) is a transcription factor with high expression in the brain. Under normal conditions, Nrf2 binds to its inhibitor Keap1 and is degraded by proteasomes. During oxidative stress, Nrf2 forms a heterodimer with Maf protein, binds to its receptor ARE, and activates the transcription of antioxidant enzymes, such as superoxide dismutase, catalase, and heme oxygenase (9, 10).
Furthermore, Nrf2 is a master regulator that can be targeted for the treatment of various diseases (11-13). The SIGMAR1 gene encodes sigma-1 receptors, which are primarily located in the endoplasmic reticulum and mitochondria-associated membranes. Sigma-1 receptors are expressed in both the peripheral and central nervous systems to modulate ionic channel functions, neuroprotection, cognition, and substance abuse (14, 15).
In addition, sigma-1 receptors also exhibit a wide range of neuroprotective mechanisms, including the regulation of endoplasmic reticulum (ER) stress via the GRP78-BiP complex and the regulation of Bcl2, an anti-apoptotic protein, by NFκB activation (16, 17). These regulatory factors may be affected by antidepressant and antioxidant agents, such as flavonoids and phenolic compounds, which are commonly found in herbal medicines (18).
2. Objectives
This study investigated the protective effects of DPP and Flv on oxidative stress induced by H2O2 in PC12 cells. PC12 cells are a commonly used model in neural biology research to assess oxidative stress (19).
3. Methods
3.1. Chemicals
The antidepressant drug Flv was acquired from Tehran Darou Pharmaceutical Company (Iran). High-glucose Dulbecco's Modified Eagle Medium (DMEM), fetal bovine serum (FBS), penicillin/streptomycin (100 U/mL), and trypsin-EDTA (0.05%, 1X) were procured from Bio Idea (Iran). Merck (Germany) provided dimethyl sulfoxide (DMSO) and hydrogen peroxide. Moreover, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was obtained from Sigma (USA). The TRIzol® reagent was acquired from GeneAll Biotechnology Co. (South Korea). The DNA synthesis and quantitative real-time polymerase chain reaction (PCR) were carried out using BioFACT™ 2X real-time PCR master mix and pre-mix contents.
3.2. Preparation of DPP and Extraction
Date palm (Phoenix dactylifera L.) pollen was collected from Bushehr province (Iran), which lies 18 meters above sea level. It is situated between a longitude of 50° 48' 59.99" E and a latitude of 28° 58' 59.99" N. The identification and confirmation of DPP were conducted by Dr. F. Ghahremaninejad. A voucher specimen of 25556 was deposited at the Herbarium of the Plant Department, Kharazmi University, Tehran, Iran.
For extraction, the grains were rinsed with deionized water, sieved, and dried. The dried powder (10 g) was dissolved in warm deionized water (100 mL at room temperature), shaken for 5 hours, filtered, and evaporated under 55 - 60°C. Finally, the prepared aqueous extract was dissolved in PBS to prepare the required doses. The stock of the DPP extract was stored at 4°C (20).
3.3. Cell Culture, Experimental Design, and Treatment
PC12 cells were cultured in DMEM containing 10% FBS and 1% antibiotic in a humidified incubator (5% CO2 at 37°C). The experimental design was performed using a modified method by Ma et al. (21). Four experimental conditions were investigated. In all cases, untreated cells were considered as the vehicle control. In the first, second, and third conditions, PC12 cells were treated with DPP extract (10 - 1000 µg/mL), H2O2 (10 - 1000 μM), and Flv (10 - 1000 μM) for 24 hours, respectively. In the fourth condition, PC12 cells were pretreated with the desired concentrations of DPP or Flv for 24 hours and then exposed to 100 µM H2O2 for 4 hours.
3.4. Cell Viability Assay
The toxicity of H2O2 (10 - 1000 μM), DPP (10 - 1000 μg), and Flv (10 - 1000 μM) in PC12 cells was assessed using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (22). For DPP and Flv exposure, PC12 cells were cultured at a density of 2×104 cells/per well and incubated for 24 hours. The cells were pretreated with DPP (200 and 500 µg/mL) or Flv (10 and 25 µM) for 24 hours and then exposed to 100µM H2O2 to assess the protective effect against oxidative stress. After the treatment, the medium was replaced with 30 µM of MTT (5 mg/mL). After 3 hours, 100 µL DMSO was added to dissolve the formazan crystals. The optical density was measured at 570 nm using an ELISA reader (Epoch, USA).
3.5. Quantitative Real-time PCR
Real-time PCR was used to evaluate gene expression (23). PC12 cells were cultured at a density of 2×105 cells/per well. After treatment, the cells were lysed with 500 µL of TRIzol®. After adding 300 µL phenol/chloroform, three phases appeared. The top phase was transferred to another microtube. Finally, after centrifugation, the pellet containing the RNA was mixed with 75% ethanol. A NanoDrop was used to determine the RNA quality. Next, the cDNA was synthesized using BioFACT™ 2X real-time PCR master mix and pre-mix contains. Oligo-DT and random hexamer primers were used for cDNA synthesis. Quantitative real-time PCR was performed in the Qiagen Rotor Gene Corbett RG6000 (Germany). The full sequence of genes Nrf2, Bcl2, SIGMAR1, and GAPDH were obtained from the National Center for Biotechnology Information (NCBI) database to design primers using Primer-BLAST. The primer sequences are listed in Table 1. The ∆∆Ct method was used to calculate relative gene expression between all experimental groups.
| Genes | Primer Sequences (5'-3') | Product Size (bp) | Tm (˚C) |
|---|---|---|---|
| Nrf2 | 166 bp | ||
| F | GGACATGGAGCAAGTTTGGC | 59.75 | |
| R | TCCAGCGAGGAGATCGATGA | 60.18 | |
| Bcl2 | 120 bp | ||
| F | ATGCCTTTGTGGAACTATATGGC | 59.11 | |
| R | GGTATGCACCCAGAGTGATGC | 61.08 | |
| SIGMAR1 | 146bp | ||
| F | AGGGCACCACAAAAAGTGAG | 60.1 | |
| R | AAGTGCAAATGCCAGGGTAG | 60.1 | |
| GAPDH | 68 bp | ||
| F | AGGTCGGTGTGAACGGATTTG | 61 | |
| R | TGTAGACCATGTAGTTGAGGTCA | 61 |
Abbreviation: PCR, polymerase chain reaction.
3.6. Statistical Analysis
Statistical analysis was performed using GraphPad Prism v. 6 software. The data are presented as mean ± standard deviation (SD). A P-value of less than 0.05 was considered significant.
4. Results
4.1. Evaluation of Cytotoxicity and Morphological Analysis
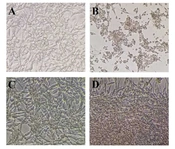
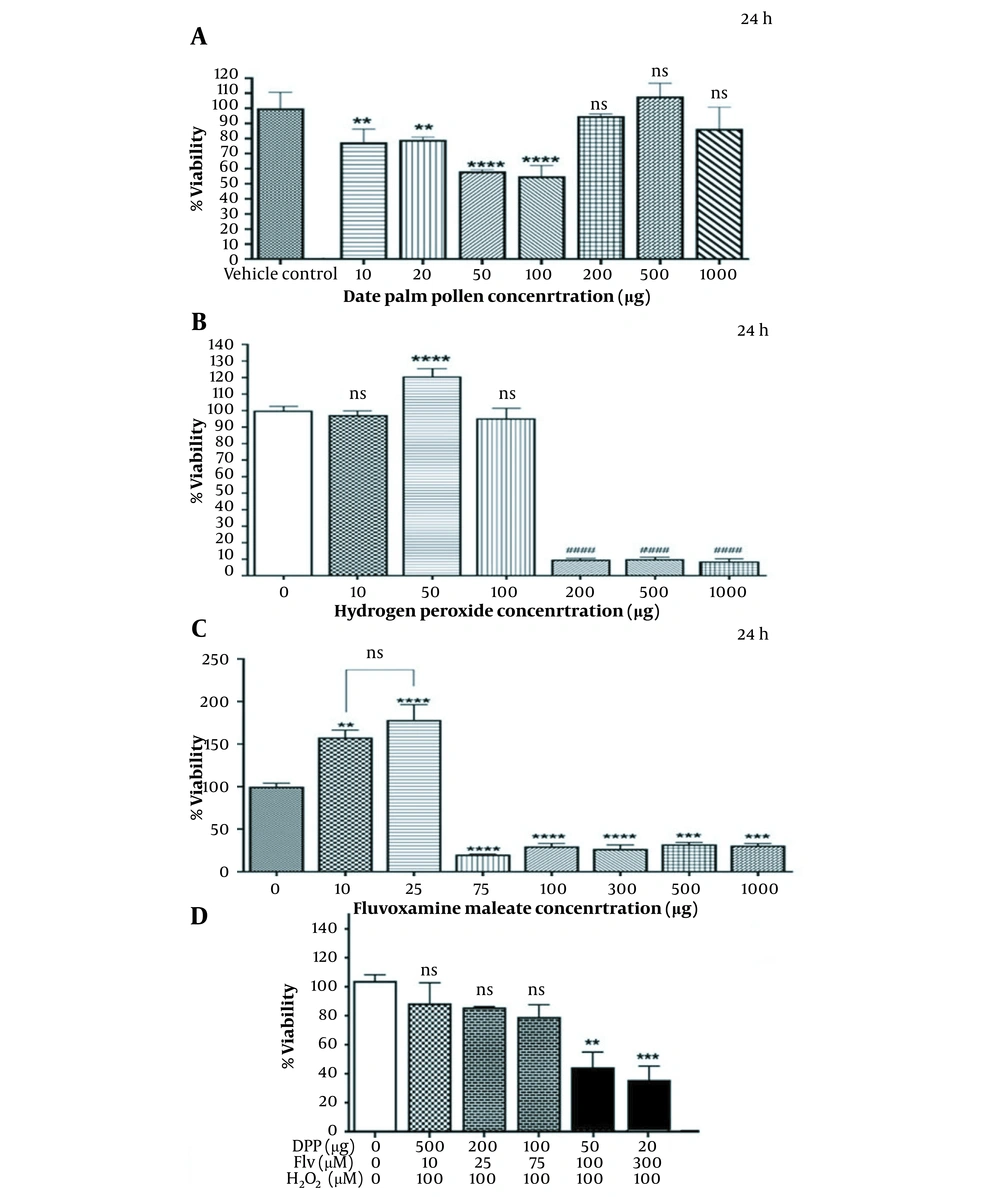
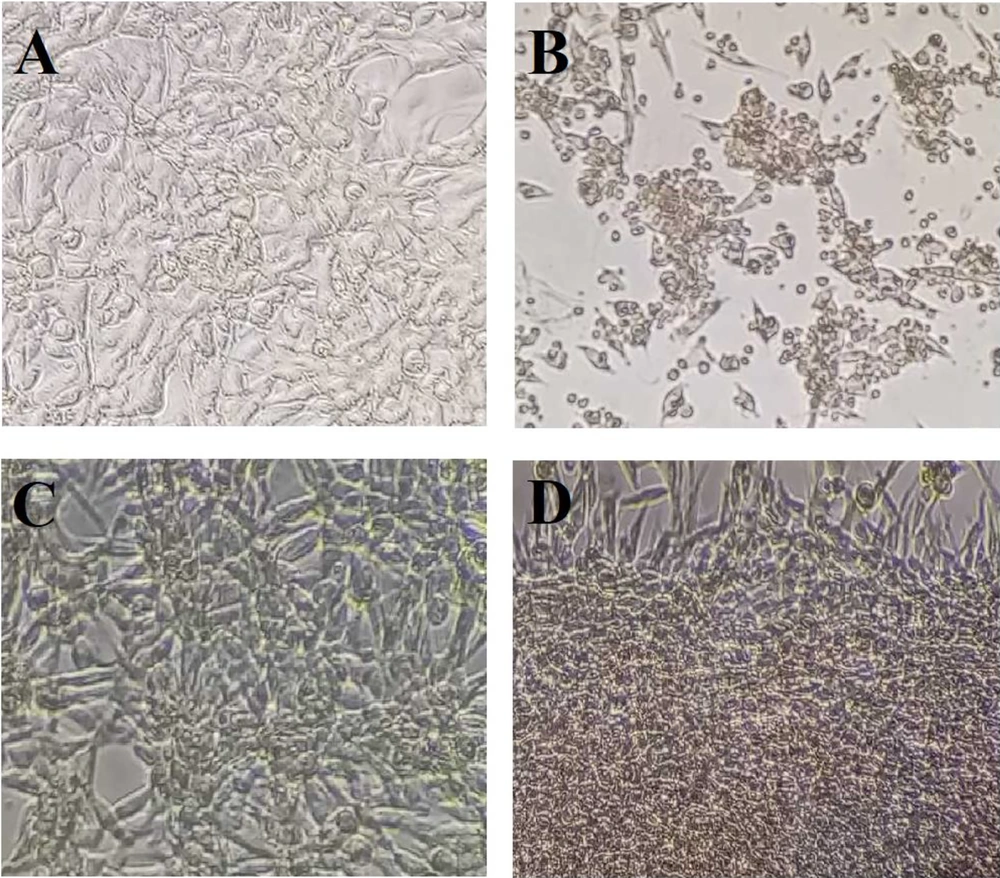
Based on the findings, DPP at concentrations of 200 - 1000 µg/mL exerted no significant effect on PC12 cells compared to the vehicle control (Figure 1A). As shown in Figure 1B and C, the viability of cells significantly decreased after incubation with H2O2 or Flv (upper dosage of 100 µM H2O2 and 25 µM Flv) in a dose-dependent manner with IC50 values of 109.5 µM and 57.26 µM, respectively. Morphological observation, such as cell shrinkage, indicated that 100 µM H2O2 was used to induce cell death and oxidative stress. As shown in Figure 1D, PC12 cells pretreated with 500 µg/mL DPP and 10 µM Flv retained their morphological features (Figure 2) against oxidative stress induced by H2O2.
Effects of DPP, Flv, and H2O2 alone or combination of DPP and Flv with H2O2 on the viability of PC12 cells. A, cell viability increased with elevating the concentrations of DPP (200 to 1000 µg/mL). Treatment with 500 µg/mL DPP showed no significant differences with the control; B, H2O2 lower than 100 µM was not toxic, but at 50 µM, it led to significant proliferation (P < 0.0001). For oxidative stress induction, the concentration of 100 µM was selected for the subsequent assay; C, Flv caused cell proliferation at doses of 10 µM (P = 0.0011) and 25 µM (P < 0.0001). In contrast, higher doses of Flv (75 to 1000 µM) significantly reduced PC12 cell survival; D, Effect of the combined treatment of 100 µM H2O2 plus pretreatment with increasing concentration of DPP (20 to 500 µg/mL) or Flv (10 to 300 µM) on PC12 cells. The co-treatment with 20, 50 µg/mL DPP and 100, 300 µM Flv caused significant cell toxicity (P = 0.0007). Data are presented as mean ± standard deviation. **P ≤ 0.01, ***P ≤ 0.001 were considered statistically significant. Abbreviations: DPP, date palm pollen; Flv, fluvoxamine maleate.
The microscopic images of PC12 cells were treated with different concentrations of DPP, Flv, and H2O2. (A) Vehicle control. (B) As shown, treatment with 100 µM H2O2 resulted in cell shrinkage after 4 h of exposure. (C) DPP exposure (500 µg/mL) suppressed morphological alteration induced by H2O2 on PC12 cells. (D) PC12 cells treated with 10 µM Flv inhibited cell death induced by H2O2. Scale bar, 100 µm.
4.2. Effects of DPP and Flv on SIGMAR1, Nrf2, and Bcl2 gene expression
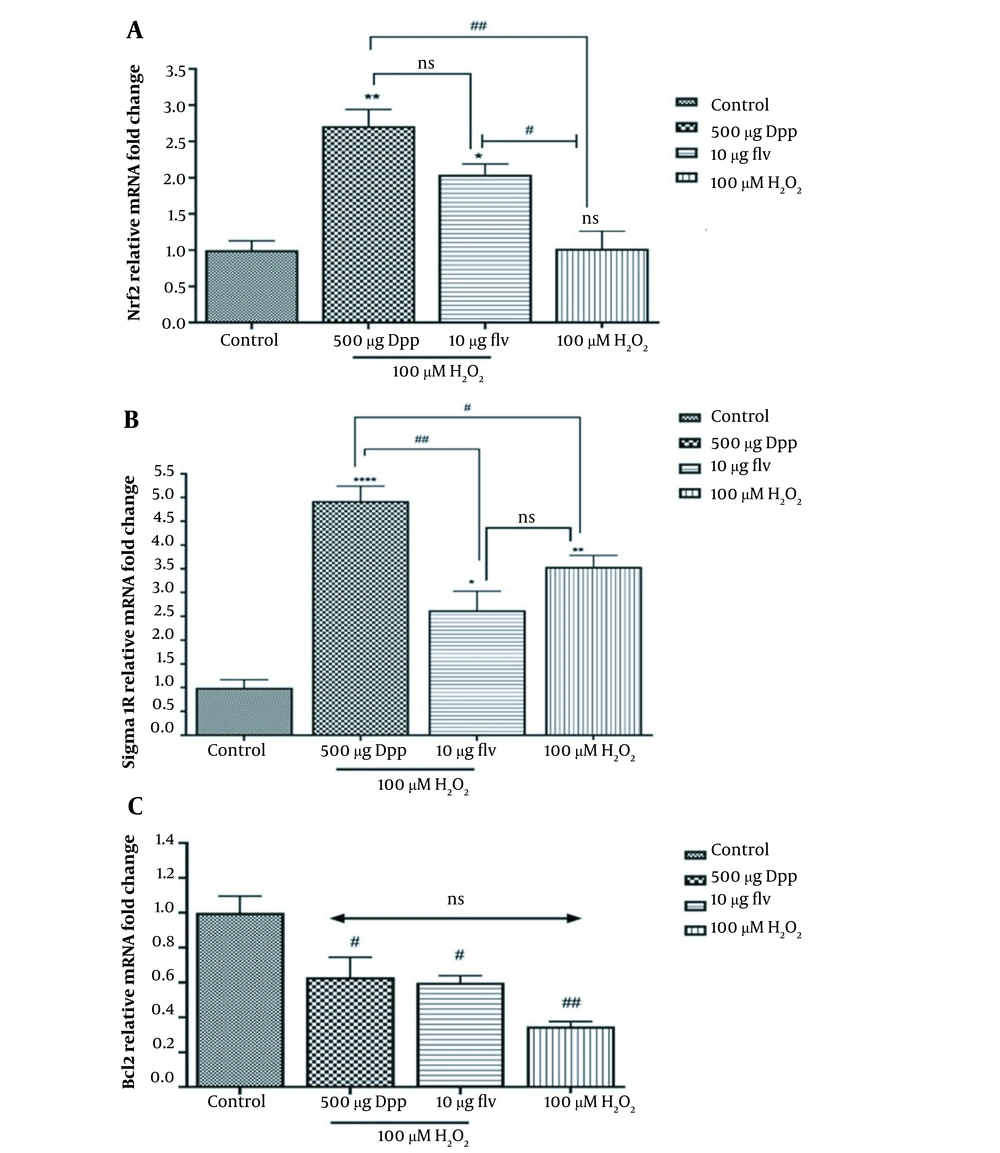
The results revealed a significant increase in Nrf2 (P = 0.0011) and SIGMAR1 (P < 0.0001) mRNA levels in PC12 cells exposed to 500 µg/mL DPP and 10 µM Flv compared to untreated cells (Figure 3A). The SIGMAR1 expression decreased in cells pretreated with 10 µM Flv (P = 0.0418) compared to cells exposed to H2O2. Therefore, Flv triggers an antioxidant response independently of ER oxidative stress regulation (Figure 3B). Bcl2 expression was downregulated in cells exposed to H2O2 compared to the vehicle control (P = 0.0294) (Figure 3C). Consequently, pretreatment with DPP and Flv (500 µg/mL and 10 µM) resulted in a small decrease in Bcl2 expression compared to the control. However, this reduction was not statistically significant compared to H2O2-exposed cells.
Effects of DPP and Flv on the expression of Nrf2, SIGMAR1, and Bcl2 at the transcriptional level. A, DPP pretreatment (500 µg/mL) elevated the level of Nrf2 rather than Flv (10 µM); B, The Flv at a concentration of 10 µM prevented PC12 cells from SIGMAR1 upregulation, and the P-value was 0.0185 compared with vehicle control. In contrast, DPP at the 500 µg/mL dose increased SIGMAR1 expression; C, DPP and Flv (500 µg/mL, 10 µM respectively) nonsignificantly elevated the Bcl2 downregulation induced by H2O2 in PC12 cells.
5. Discussion
The present study explored the protective effects of DPP and Flv against H2O2-induced oxidative damage in PC12 cells. According to findings, H2O2 below 100 μM is nontoxic. Previous studies have demonstrated that H2O2 can inhibit cell growth and cause cell death; higher doses of H2O2 can lead to oxidative stress (24). In this study, 100 μM H2O2 induced oxidative stress with less severe apoptotic effects.
The results revealed that DPP was nontoxic to PC12 cells and even induced slight cell growth at a concentration of 500 μg/mL compared to the vehicle control. While a previous study has demonstrated the neuroprotective potential of DPP (25), research into its effects on the nervous system and the expression of neural oxidative stress-related genes remains limited. As previously reported, DPP exhibits antioxidant properties owing to the presence of phytochemical agents, including various flavonoids and unsaturated fatty acids (26).
Date palm pollen demonstrated neuroprotection against oxidative stress and neuronal injury induced by bilateral carotid artery occlusion (25). Recently, DPP has been shown to protect against doxorubicin-induced cardiomyopathy and hepatotoxicity, both of which are disorders associated with oxidative stress (4, 27).
Regarding the neuroprotective effect of Flv, doses of 10 μM and 25 μM increased cell growth, but the difference between these doses was not statistically significant. Oxidative stress has been recognized as a crucial factor in depression. However, previous studies have documented the combined antioxidant and antidepressant effects of antidepressants such as fluvoxamine (28, 29). Schlezinger et al. reported that Flv inhibits ROS generation by suppressing the cytochrome P450 family 1 subfamily A member 2 (CYP1A2) enzyme, which is responsible for metabolizing antioxidants in the body (30).
Evaluation of the protective effects of DPP and Flv showed a significant increase in Nrf2 expression in DPP and Flv-pretreated cells (500 μg/mL and 10 μM, respectively). H2O2 induces oxidative stress and influences adaptive responses, such as the Nrf2 pathway. Various studies have identified Nrf2 as a guardian of redox homeostasis (9). The expression level of Nrf2 in the DPP group was higher than that in the H2O2 group, indicating that DPP and Flv prevent the initiation of oxidative stress primarily through the Nrf2 pathway.
Previous studies have shown that forsythiaside provided protection against neurotoxicity through the elevation of Nrf2 levels and the upregulation of SOD and CAT (31). In 2018, it was reported that duloxetine could protect neuroblastoma cells primarily through Nrf2 upregulation and the expression of HO-1, a target gene of Nrf2 (32). Kolla et al. reported findings that amitriptyline and fluoxetine exerted neuroprotective effects on PC12 cells exposed to H2O2, which was associated with upregulation in SOD activity (7). The sigma-1 receptor was chosen because it has been shown that fluvoxamine maleate (Flv) binds to it, so being an agonist of the sigma-1 receptor would be congruent with our antidepressant selection (33). The sigma-1 receptor agonist's fluvoxamine, fluoxetine, and citalopram bind to the ER sigma-1 receptors and cause them to separate from the complex with BiP (GRP78). Further, dissociating the sigma-1 receptor will allow it to generate chaperone activity, resulting in neuroprotection (15).
According to our findings, SIGMAR1 expression increased in all experimental states compared with the control. Treatment with DPP (at 500 µg/mL) significantly impacted SIGMAR1 expression compared to H2O2-exposed cells. This finding suggested that DPP could have a more significant impact on the Nrf2 pathway than the ER pathway. In contrast, Flv slightly attenuated the expression of SIGMAR1 in H2O2-exposed cells, indicating the role of Flv via regulation of ER oxidative stress.
Previous studies have demonstrated the association between sigma-1 receptor dysfunction and depression, suggesting its potential as an antidepressant target (17). Additionally, studies have shown that sigma-1 receptor agonists can exert neuroprotection against neurotoxicity in neuronal cell lines (34). Recently, the antifibrotic efficacy of fluvoxamine has been confirmed in a vascular disorder associated with oxidative damage (35).
The role of oxidative stress and mitochondrial dysfunction through apoptosis in neurodegenerative disease has been studied previously (35). It was elucidated that oxidative stress exerts ferroptosis and mitochondrial dysregulation, which lead to neuronal cell death and apoptosis (2). The expression of Bcl2, an antiapoptotic factor from the Bcl2 family, was found to be significantly attenuated in H2O2-treated cells compared to the control. However, pretreatment with DPP and Flv reduced Bcl2 gene expression, but this downregulation did not show any significant difference between the experimental groups.
Nakayama et al. demonstrated the neuroprotection of ferulic acid as a phytochemical against H2O2-induced apoptosis. Ferulic acid increased the expression of BDNF, a neuroprotective factor, and regulated the activity of phosphokinases and apoptosis-related proteins (23). Additionally, quercetin has been shown to protect PC12 cells from H2O2-induced neurotoxicity by elevating the SOD and CAT level, reducing the apoptosis markers, such as Bax and caspase-3, and upregulating the Bcl2 (22).
The combined suppression of oxidative stress induced by H2O2 by DPP and Flv through the Nrf2 and sigma-1 signaling pathways suggests that these pretreatment packages could be an effective modality for preventing neurodegenerative damage in an animal neurotoxicity model.