Abstract
Keywords
Diabetes Mellitus Grape Seeds Extract Blood Pressure Clinical Trial
1. Background
Diabetes mellitus (DM) is a widespread chronic metabolic disease characterized by an increase in blood glucose levels. It has two types: Type 1 (T1DM) and Type 2 (T2DM). Type 1DM, which results from an autoimmune response, typically begins in youth and is associated with an absolute deficiency of insulin. Type 2DM, more prevalent than T1DM (1), is characterized by insulin resistance and hyperinsulinemia.
According to the final report from the Centers for Disease Control and Prevention (CDC), more than 130 million adults in the United States are living with diabetes or prediabetes (2). In Iran, the prevalence of diabetes and prediabetes was reported to be 15% and 25.4%, respectively, between 2014 and 2020, with only 41.2% of treated cases being effectively controlled (3). Type 2DM and its complications can significantly reduce lifespan and impose an enormous financial burden on healthcare systems due to the high costs of care and management. Consequently, developing novel methods to enhance glucose control and reduce the risk of complications, such as alternative medicinal foods, could be extremely beneficial (4).
Alternative medicine is increasingly popular today, with scientists searching for natural remedies that can help humanity lead better and healthier lives (5). There are reports that natural bioactive compounds may control blood glucose levels and reduce the rate of complications (1).
Grapes (Vitis Vinifera L.) are a well-known fruit cultivated in many countries worldwide and are believed by many cultures to have beneficial medical effects (6). Native to the Mediterranean region, southwest Asia, and central Europe, grapes are a rich source of sugars, carbohydrates, proteins, lipids, vitamins, minerals, and bioactive compounds such as quercetin, resveratrol, and epigallocatechin-3-gallate (EGCG). These polyphenolic compounds are known to promote human health (7, 8). Grape seeds, a byproduct of grapes, have numerous applications in the food industry. They are particularly rich in polyphenols, mainly proanthocyanidins, and possess the highest total phenolic content (TPC) compared to grape skin and pulp, although they contain almost no anthocyanins. Flavan-3-ols are the most abundant flavonoids in grape seeds, and flavonols, including quercetin, myricetin, and kaempferol, can also be found in the seeds of some grape varieties (9).
Numerous studies have demonstrated the various medical benefits of grape seed in both humans and animals, including anti-tumor (10), lipid-lowering (11), anti-atherosclerotic (12), neuroprotective (13), gut microbiota-modulating (14), and antioxidant effects (15).
Research indicates that grape seed extract (GSE) can improve metabolic conditions in patients, including enhancements in insulin concentration and resistance (16), diastolic (17, 18) and systolic blood pressure (BP) (18), and total cholesterol levels (19). Moreover, several studies have validated the role of grape seed and its constituents in ameliorating diabetic complications such as retinopathy (20), nephropathy (21, 22), and neuropathy (23), as well as in reducing systolic or diastolic BP (24).
To the best of our knowledge, few studies have investigated the effect of Iranian GSE on metabolic parameters in patients with pure T2DM (25), and none have assessed such effects in Iran using Iranian GSE. Only one research study has been conducted with Iranian GSE on T2DM, focusing on antioxidant status (26).
2. Objectives
Therefore, in this study, we aim to explore the impact of Iranian GSE on fasting blood sugar (FBS), lipid profile, and BP in patients with T2DM.
3. Methods
Patients with T2DM referred to the Department of Endocrinology at Golestan Hospital, Ahvaz, Iran, were screened and enrolled based on criteria established by the American Diabetes Association (ADA). According to the ADA criteria, individuals with fasting plasma glucose (FPG) ≥ 126 mg/dL (7.0 mmol/L) are considered diabetic (27). Participants were aged between 25 and 80 years and were receiving oral hypoglycemic agents.
Exclusion criteria included a history of T2DM diagnosis of more than ten years, treatment with insulin, severe hepatic insufficiency (alanine aminotransferase (ALT) more than 100 U/I), severe renal insufficiency (glomerular filtration rate (GFR) less than 30 mL/min/1.73 m2), any type of allergies, severe cardiovascular or hematologic disorders, and pregnant or lactating women.
Grape seed capsules were sourced from Shari Nutraceutical Company, Tehran, Iran. Each capsule contained 263.2 mg of GSE standardized to contain 250 mg of proanthocyanidin, equivalent to 5.3 g of grape seed. The product was obtained from vineyards in the northwest of Iran.
Eighty patients with T2DM entered the study. Eligible patients were randomly assigned to either the GSE (treatment) group or the placebo (control) group. A permuted-block randomization method with a block size of 4 and an allocation ratio of 1:1 was used. Each participant received a code for allocation concealment, and these codes were kept in opaque sealed envelopes by the clinic's secretary until the end of the intervention.
The demographic details of the patients, history of smoking, medications, and medical history were recorded. Measurements of height, weight, Body Mass Index (BMI), and circumferences of the hip and waist were also taken. The BMI was calculated as body weight in kilograms divided by the square of height in meters. These five size parameters were recorded before and at the end of the study to rule out any bias.
Both groups were instructed to take the capsules orally every 12 hours before meals with a glass of water for 30 days. The treatment group received GSE capsules (263.2 mg GSE twice daily). The placebo group received capsules identical in shape, color, and weight to the GSE capsules but without the active ingredient (GSE), and these were produced by the same manufacturer.
To control for external variables, patients were asked to maintain their previous diet and exercise regimen throughout the study period. They were reminded of this every weekend. At the beginning and end of the study, fasting blood samples were drawn from the antecubital vein after nine hours of fasting to assess changes in biochemical parameters. Blood pressure was also measured at these times.
3.1. Ethical Affairs
Volunteers signed a written informed consent during a face-to-face meeting where all information about the study was explained. Both the supplements and placebo were approved by the Iranian Ministry of Health and Medical Education. The study protocols were confirmed by the Ethics Committee of Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran (case number: IR.AJUMS.HGOLESTAN.REC.1400.012), and by the official organization "The Iranian Registry of Clinical Trials, IRCT," a branch of the Iranian Ministry of Health and Medical Education (code: "IRCT20210612051544N1"). The authors declare that there have been no changes to the protocols recorded on this website.
3.2. Biochemical Assays
Biochemical analyses were performed both before and at the end of the intervention at the Diabetes Research Center, Health Research Institute, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran. The levels of serum glucose, triglycerides (TG), total cholesterol, low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C) were measured according to the manufacturer's instructions using a commercial kit from Parsazmoon, Iran, and a multi-analyzer (BT 3000, Italy).
3.3. Blood Pressure Measurement
Systolic and diastolic BP were measured using the appropriate cuff size on the bare arm at heart level and in a sitting position at the beginning and the end of the study, using a mercurial sphygmomanometer (YAMASU, Japan). During the measurement, patients were required to remain silent, with an empty bladder and legs uncrossed. Additionally, their back and feet were supported in accordance with the ACC/AHA hypertension guidelines (28).
3.4. Statistical Analysis
Quantitative data were presented as means ± standard deviations (SD), and qualitative data as frequencies and percentages. The chi-square test was used to compare qualitative variables between the two groups. Quantitative variables were compared using the independent t-test or its nonparametric equivalent, the Mann-Whitney U test. Analysis of covariance (ANCOVA) was also employed to evaluate the treatment effect. Statistical analysis was conducted using SPSS version 22 for Windows (SPSS Inc., Chicago, IL). P-values less than 0.05 were considered significant.
4. Results
A total of 80 patients were enrolled in the trial between April and September 2021. They were randomly divided into two groups: The treatment (GSE) group (n = 40) and the placebo group (n = 40). Two patients from the treatment group were excluded mid-study due to irregular drug intake. Four patients from the placebo group were also excluded; one passed away in a car accident, one reported not adhering to his routine regimen and consuming excessive sugar in the last week of the study, and two contracted COVID-19 and stopped taking their capsules on their own. Consequently, 74 patients completed the study and were included in the analysis (Figure 1).
Study plot
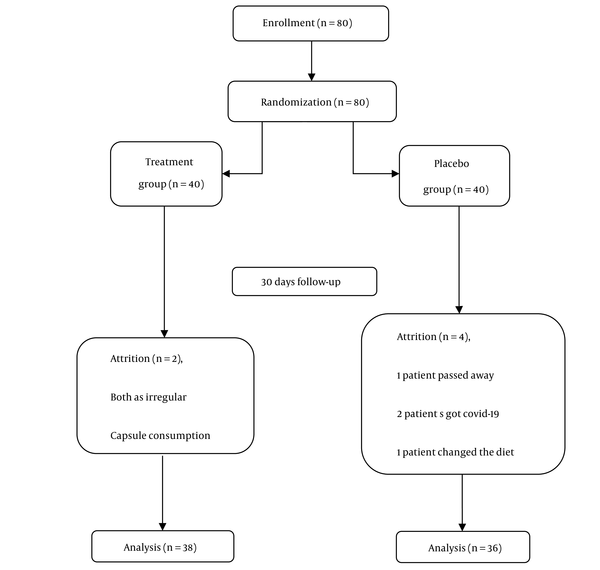
The demographics and general characteristics of these 74 patients are presented in Table 1. The mean age, height, weight, waist circumference, hip circumference, BMI, number of smokers, and the proportion of males and females were comparable between the two groups. The oral hypoglycemic drugs used during this trial showed no statistically significant difference between the groups, except for Synoripa (empagliflozin/metformin) tablets. The number of patients taking Synoripa in the treatment group was significantly higher than in the placebo group (P = 0.01). Additionally, there were no significant differences between the baseline systolic/diastolic BP, FBS, and lipid profile elements, including TG, total cholesterol, high-density lipoprotein (HDL), and low-density lipoprotein (LDL) (Table 2). No changes in diet or physical activity were prescribed, as confirmed in the final interview at the end of the study.
| Variables | Treatment (n = 38) | Placebo (n = 36) | P-Value |
|---|---|---|---|
| Age 1, y | 52.89 ± 10.01 | 53.36 ± 10.63 | 0.85 |
| Sex 3, male/female | 18/20 | 14/22 | 0.49 |
| Height 1,cm | 168.7 ± 8.77 | 167.66 ± 11.31 | 0.66 |
| Waist 1, cm | 101.5 ± 11.07 | 99.84 ± 6.89 | 0.47 |
| Weight 2, kg | 80.81 ± 11.38 | 78.72 ± 9.67 | 0.69 |
| Hip 2, cm | 111.68 ± 14.11 | 114.29 ± 10.41 | 0.37 |
| BMI 1, kg/m2 | 28.22 ± 4.04 | 28.09 ± 3.85 | 0.89 |
| Smoking 3 −/+ | 36/2 | 34/2 | 0.96 |
| Synoripa 3 −/+ | 38/0 | 29/7 | 0.01 b |
| Zipmet 3 −/+ | 17/21 | 18/18 | 0.65 |
| Acarbose 3 −/+ | 32/6 | 32/4 | 0.74 |
| Metformin 3 −/+ | 16/22 | 21/15 | 0.16 |
| Empagliflozine 3 −/+ | 38/0 | 35/1 | 0.98 |
| Glibenclamide 3 −/+ | 27/11 | 22/14 | 0.37 |
4.1. Effect of Iranian Grape Seed Extract on Fasting Blood Sugar (FBS) & Lipid Profile
Fasting blood sugar, total cholesterol, HDL, LDL, and TG were measured on day 0 and day 30 for both groups, as shown in Table 2. At the end of the intervention, the mean FBS in the treatment group significantly decreased by 10.2% from 144.75 ± 30.82 in the placebo group to 129.87 ± 31.79 (P = 0.001). There were no significant differences between the two groups regarding mean total cholesterol, HDL, LDL, and TG after the intervention with GSE consumption.
Effect of Iranian Grape Seed Extract on Lipid Profile, Blood Pressure, and Fasting Blood Sugar in Type 2DM Patients After 30 Days a, b
| Variables | Treatment (n = 38) | Placebo (n = 36) | P-Value (Before) | P-Value (After) | ||
|---|---|---|---|---|---|---|
| Before | After | Before | After | |||
| DBP, mmHg | 85.78 ± 9.69 | 82.57 ± 6.41 | 85.27 ± 9.71 | 83.89 ± 7.57 | 0.82 | 0.14 |
| SBP, mmHg | 126.84 ± 15.26 | 121.94 ± 7.49 | 124.17 ± 16.28 | 125.8 ± 13.39 | 0.47 | 0.002 c |
| FBS, mg/dL | 148.92 ± 22.2 | 129.87 ± 31.79 | 145.94 ± 22.56 | 144.75 ± 30.82 | 0.57 | 0.001 c |
| Total cholesterol, mg/dL | 152.87 ± 40.63 | 148.13 ± 38.28 | 144.03 ± 35.18 | 147.94 ± 34.27 | 0.32 | 0.35 |
| HDL, mg/dL | 43.3 ± 9.8 | 44.59 ± 10.92 | 39.6 ± 8.58 | 40.25 ± 9.26 | 0.85 | 0.37 |
| LDL, mg/dL | 79.97 ± 27.42 | 75.08 ± 25.73 | 77.2 ± 29.39 | 73.8 ± 26.14 | 0.69 | 0.88 |
| TG, mg/dL | 157.05 ± 80.59 | 154.79 ± 72.25 | 143.36 ± 63.76 | 148.7 ± 61.59 | 0.42 | 0.56 |
4.2. Effect of Iranian Grape Seed Extract on Systolic & Diastolic Blood Pressure
On day 0 and day 30, systolic and diastolic BP were recorded, as shown in Table 2. At the end of the intervention, there was a statistically significant decrease of 3% in mean SBP from 125.83 ± 13.39 in the placebo group to 121.94 ± 7.49 in the treatment group (P = 0.002).
5. Discussion
Diabetes mellitus is a common chronic disease characterized by elevated blood glucose levels, leading to various complications such as neuropathy, nephropathy, retinopathy, and diabetic foot ulcers. Each of these complications can cause significant morbidity and impose a substantial financial burden on the healthcare system (1, 4). Therefore, strict management of blood glucose levels is a critical treatment objective. Around the world, grape seeds have been utilized for various health-enhancing purposes (6).
In this study, we aimed to investigate the short-term effects of Iranian GSE on FBS, lipid profile, and BP in patients with T2DM. After one month of treatment with Iranian GSE, there was a significant reduction in FBS in the treatment group compared to the placebo. Acharya et al. also demonstrated a similar effect in patients with T2DM after three months, although the exact dose of GSE used was not specified (25). Irandoost et al. reported no significant effect on FBS after eight weeks of supplementation with grape seed oil (GSO), which may be due to the negligible amount of proanthocyanidins in GSO (29, 30), suggesting that the FBS-lowering effect of GSE may be attributed to proanthocyanidins. Park et al. found that a beverage containing GSE (300 mg/day) did not affect FBS after six weeks in pre-hypertension volunteers (31). Although Park's study was longer, the lack of significant change could be due to the lower dosage of GSE compared to our study (526.4 mg/day).
As presented in Table 1, there were no significant differences in the baseline characteristics of both groups, except for the use of Synoripa. The number of patients who received Synoripa in the treatment group was significantly higher than in the placebo group. Synoripa, a combination of metformin and empagliflozin—both glucose-lowering drugs—may introduce considerable bias into our study. Lin et al. showed that combining metformin with dapagliflozin, a drug similar to empagliflozin as both inhibit the sodium-glucose transporter-2 (SGLT-2), did not result in significant changes in FBS or HbA1c compared to metformin alone (32), suggesting that metformin and empagliflozin do not have a synergistic effect on lowering blood glucose, which should not impact the results of this study.
In this study, we observed a significant decrease in systolic BP after one month of treatment with GSE. Biesinger in 2016 and Sivaprakasapillai in 2009 conducted research of the same duration as the current study and both reported positive effects on reducing BP (17, 18). Biesinger et al. found that consuming 330 mg/day of GSE for 28 days significantly reduced diastolic BP (17). Sivaprakasapillai et al. reported that taking GSE in two doses (150 & 300 mg/day) lowered both diastolic and systolic BP (18). Three factors may explain the varying effects of GSE on diastolic and systolic BP across these studies and ours; although all studies were of the same duration, the other two trials used a different type of GSE (sourced from the USA) in lesser quantities compared to our study, and the volunteers in the other trials were hypertensive, whereas our study did not categorize patients based on BP. It appears that hypertensive patients responded better to lower doses of GSE compared to a mixed group of hypo-, hyper-, and normotensive patients. Interestingly, Park et al. also showed that the greater the BP, the more pronounced the effect of GSE on reducing BP (31).
In the current study, there was no significant change in any element of the lipid profile (total cholesterol, HDL, LDL, and TG). In contrast, a study by Yousefi et al. in 2021 demonstrated significant improvements in levels of HDL-C, LDL-C, total cholesterol, and TG when GSE (300 mg/day) was administered for 12 weeks. They also used Iranian GSE, albeit in a smaller quantity compared to this study (33). It is recommended to investigate the effects of Iranian GSE over a longer period in clinical trials.
5.1. Conclusions
To conclude, consuming Iranian GSE over a short period can reduce FBS and systolic BP. The glucose-lowering effect of GSE may be attributable to the presence of proanthocyanidins. Therefore, GSE could play a crucial role in enhancing BP and fasting blood glucose management in patients with type 2 diabetes mellitus.
Acknowledgements
References
-
1.
Zakerkish M, Jenabi M, Zaeemzadeh N, Hemmati AA, Neisi N. The Effect of Iranian Propolis on Glucose Metabolism, Lipid Profile, Insulin Resistance, Renal Function and Inflammatory Biomarkers in Patients with Type 2 Diabetes Mellitus: A Randomized Double-Blind Clinical Trial. Sci Rep. 2019;9(1):7289. [PubMed ID: 31086222]. [PubMed Central ID: PMC6514000]. https://doi.org/10.1038/s41598-019-43838-8.
-
2.
Centers for Disease Control and Prevention. National Diabetes Statistics Report website. Mosques, Malaysia: Centers for Disease Control and Prevention; 2022, [cited 2023]. Available from: https://www.cdc.gov/diabetes/data/statistics-report/index.html.
-
3.
Khamseh ME, Sepanlou SG, Hashemi-Madani N, Joukar F, Mehrparvar AH, Faramarzi E, et al. Nationwide Prevalence of Diabetes and Prediabetes and Associated Risk Factors Among Iranian Adults: Analysis of Data from PERSIAN Cohort Study. Diabetes Ther. 2021;12(11):2921-38. [PubMed ID: 34595726]. [PubMed Central ID: PMC8521563]. https://doi.org/10.1007/s13300-021-01152-5.
-
4.
Li Y, Chen M, Xuan H, Hu F. Effects of encapsulated propolis on blood glycemic control, lipid metabolism, and insulin resistance in type 2 diabetes mellitus rats. Evid Based Complement Alternat Med. 2012;2012:981896. [PubMed ID: 21716678]. [PubMed Central ID: PMC3118452]. https://doi.org/10.1155/2012/981896.
-
5.
Shah M, Alharby HF, Hakeem KR. Lantana camara: A Comprehensive Review on Phytochemistry, Ethnopharmacology and Essential Oil Composition. Letter Appl NanoBioSci. 2020;9(3):1199-207. https://doi.org/10.33263/lianbs93.11991207.
-
6.
Akaberi M, Hosseinzadeh H. Grapes (Vitis vinifera) as a Potential Candidate for the Therapy of the Metabolic Syndrome. Phytother Res. 2016;30(4):540-56. [PubMed ID: 26800498]. https://doi.org/10.1002/ptr.5570.
-
7.
Majeed U, Shafi A, Majeed H, Akram K, Liu X, Ye J, et al. Grape (Vitis vinifera L.) phytochemicals and their biochemical protective mechanisms against leading pathologies. Food Chemistry. 2023;405:134762. https://doi.org/10.1016/j.foodchem.2022.134762.
-
8.
McGill CR, Keast DR, Painter JE, Romano CS, Wightman JD. Improved diet quality and increased nutrient intakes associated with grape product consumption by U.S. children and adults: National Health and Nutrition Examination Survey 2003 to 2008. J Food Sci. 2013;78 Suppl 1:A1-4. [PubMed ID: 23789930]. https://doi.org/10.1111/1750-3841.12066.
-
9.
Zhou DD, Li J, Xiong RG, Saimaiti A, Huang SY, Wu SX, et al. Bioactive Compounds, Health Benefits and Food Applications of Grape. Foods. 2022;11(18). [PubMed ID: 36140883]. [PubMed Central ID: PMC9497968]. https://doi.org/10.3390/foods11182755.
-
10.
Zhu F, Du B, Li J. Recent advance on the antitumor and antioxidant activity of grape seed extracts. Int J Wine Res. 2015;7:63—67. https://doi.org/10.2147/ijwr.s76162.
-
11.
Moreno DA, Ilic N, Poulev A, Brasaemle DL, Fried SK, Raskin I. Inhibitory effects of grape seed extract on lipases. Nutr. 2003;19(10):876-9. [PubMed ID: 14559324]. https://doi.org/10.1016/s0899-9007(03)00167-9.
-
12.
Curin Y, Ritz MF, Andriantsitohaina R. Cellular mechanisms of the protective effect of polyphenols on the neurovascular unit in strokes. Cardiovasc Hematol Agents Med Chem. 2006;4(4):277-88. [PubMed ID: 17073605]. https://doi.org/10.2174/187152506778520691.
-
13.
Charradi K, Elkahoui S, Karkouch I, Limam F, Hassine FB, Aouani E. Grape seed and skin extract prevents high-fat diet-induced brain lipotoxicity in rat. Neurochem Res. 2012;37(9):2004-13. [PubMed ID: 22684284]. https://doi.org/10.1007/s11064-012-0821-2.
-
14.
Ferreira YAM, Jamar G, Estadella D, Pisani LP. Proanthocyanidins in grape seeds and their role in gut microbiota-white adipose tissue axis. Food Chem. 2023;404(Pt A):134405. [PubMed ID: 36444031]. https://doi.org/10.1016/j.foodchem.2022.134405.
-
15.
Bagchi D, Bagchi M, Stohs SJ, Das DK, Ray SD, Kuszynski CA, et al. Free radicals and grape seed proanthocyanidin extract: importance in human health and disease prevention. Toxicol. 2000;148(2-3):187-97. [PubMed ID: 10962138]. https://doi.org/10.1016/s0300-483x(00)00210-9.
-
16.
Alizadeh M, Taghizadeh SKS. Effect of 8 weeks' supplementation grape seed extract on insulin resistance in iranian adolescents with metabolic syndrome: A randomized controlled trial. Diabetes Metab Syndr. 2021;15(1):197-203. [PubMed ID: 33385766]. https://doi.org/10.1016/j.dsx.2020.12.028.
-
17.
Biesinger S, Michaels HA, Quadros AS, Qian Y, Rabovsky AB, Badger RS, et al. A combination of isolated phytochemicals and botanical extracts lowers diastolic blood pressure in a randomized controlled trial of hypertensive subjects. Eur J Clin Nutr. 2016;70(1):10-6. [PubMed ID: 26059745]. https://doi.org/10.1038/ejcn.2015.88.
-
18.
Sivaprakasapillai B, Edirisinghe I, Randolph J, Steinberg F, Kappagoda T. Effect of grape seed extract on blood pressure in subjects with the metabolic syndrome. Metab. 2009;58(12):1743-6. [PubMed ID: 19608210]. https://doi.org/10.1016/j.metabol.2009.05.030.
-
19.
Kar P, Laight D, Rooprai HK, Shaw KM, Cummings M. Effects of grape seed extract in Type 2 diabetic subjects at high cardiovascular risk: a double blind randomized placebo controlled trial examining metabolic markers, vascular tone, inflammation, oxidative stress and insulin sensitivity. Diabet Med. 2009;26(5):526-31. [PubMed ID: 19646193]. https://doi.org/10.1111/j.1464-5491.2009.02727.x.
-
20.
Moon SW, Shin YU, Cho H, Bae SH, Kim HK, and for the Mogen Study G. Effect of grape seed proanthocyanidin extract on hard exudates in patients with non-proliferative diabetic retinopathy. Med (Baltimore). 2019;98(21). e15515. [PubMed ID: 31124931]. [PubMed Central ID: PMC6571433]. https://doi.org/10.1097/MD.0000000000015515.
-
21.
Mansouri E, Panahi M, Ghaffari MA, Ghorbani A. Effects of grape seed proanthocyanidin extract on oxidative stress induced by diabetes in rat kidney. Iran Biomed J. 2011;15(3):100-6. [PubMed ID: 21987116]. [PubMed Central ID: PMC3639749].
-
22.
Li X, Xiao Y, Gao H, Li B, Xu L, Cheng M, et al. Grape seed proanthocyanidins ameliorate diabetic nephropathy via modulation of levels of AGE, RAGE and CTGF. Nephron Exp Nephrol. 2009;111(2):e31-41. [PubMed ID: 19142024]. https://doi.org/10.1159/000191103.
-
23.
Ding Y, Dai X, Jiang Y, Zhang Z, Li Y. Functional and morphological effects of grape seed proanthocyanidins on peripheral neuropathy in rats with type 2 diabetes mellitus. Phytother Res. 2014;28(7):1082-7. [PubMed ID: 24343984]. https://doi.org/10.1002/ptr.5104.
-
24.
Grohmann T, Litts C, Horgan G, Zhang X, Hoggard N, Russell W, et al. Efficacy of Bilberry and Grape Seed Extract Supplement Interventions to Improve Glucose and Cholesterol Metabolism and Blood Pressure in Different Populations-A Systematic Review of the Literature. Nutr. 2021;13(5). [PubMed ID: 34067538]. [PubMed Central ID: PMC8156535]. https://doi.org/10.3390/nu13051692.
-
25.
Acharya S, Gujjari SK, Murthy K, Battula R. Evaluation of Grape Seed Formulation as an Adjunct to Scaling and Root Planing on Oxidative Stress, Inflammatory Status and Glycaemic Control in Type 2 Diabetic Patients with Chronic Periodontitis: A Randomised Controlled Trial. J Clin Diagn Res. 2021;148(2-3):187-97. https://doi.org/10.7860/jcdr/2021/45235.14792.
-
26.
Pourghassem Gargari B, Abedini S, Babaei H, Aliasgarzadeh A, Pourabdollahi P. Effect of supplementation with grape seed (Vitis vinifera) extract on antioxidant status and lipid peroxidation in patient with type ΙΙ diabetes. J Med Plants Res. 2011;5:2029-34.
-
27.
American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33 Suppl 1(Suppl 1):S62-9. [PubMed ID: 20042775]. [PubMed Central ID: PMC2797383]. https://doi.org/10.2337/dc10-S062.
-
28.
Flack JM, Adekola B. Blood pressure and the new ACC/AHA hypertension guidelines. Trends Cardiovasc Med. 2020;30(3):160-4. [PubMed ID: 31521481]. https://doi.org/10.1016/j.tcm.2019.05.003.
-
29.
Irandoost P, Ebrahimi-Mameghani M, Pirouzpanah S. Does grape seed oil improve inflammation and insulin resistance in overweight or obese women? Int J Food Sci Nutr. 2013;64(6):706-10. [PubMed ID: 23506314]. https://doi.org/10.3109/09637486.2013.775228.
-
30.
Nakamura Y, Tsuji S, Tonogai Y. Analysis of Proanthocyanidins in Grape Seed Extracts, Health Foods and Grape Seed Oils. J Health Sci. 2003;49(1):45-54. https://doi.org/10.1248/jhs.49.45.
-
31.
Park E, Edirisinghe I, Choy YY, Waterhouse A, Burton-Freeman B. Effects of grape seed extract beverage on blood pressure and metabolic indices in individuals with pre-hypertension: a randomised, double-blinded, two-arm, parallel, placebo-controlled trial. Br J Nutr. 2016;115(2):226-38. [PubMed ID: 26568249]. https://doi.org/10.1017/S0007114515004328.
-
32.
Lin YY, Weng SF, Hsu CH, Huang CL, Lin YP, Yeh MC, et al. Effect of metformin monotherapy and dual or triple concomitant therapy with metformin on glycemic control and lipid profile management of patients with type 2 diabetes mellitus. Front Med (Lausanne). 2022;9:995944. [PubMed ID: 36314019]. [PubMed Central ID: PMC9614085]. https://doi.org/10.3389/fmed.2022.995944.
-
33.
Yousefi R, Parandoosh M, Khorsandi H, Hosseinzadeh N, Madani Tonekaboni M, Saidpour A, et al. Grape seed extract supplementation along with a restricted-calorie diet improves cardiovascular risk factors in obese or overweight adult individuals: A randomized, placebo-controlled trial. Phytother Res. 2021;35(2):987-95. [PubMed ID: 33044768]. https://doi.org/10.1002/ptr.6859.

