1. Background
In the United States, 25% of deaths were due to cancer from 2008 to 2010 (1-3). Also, it has been reported that the risk of being diagnosed with cancer for the Americans is 41% (4). Therefore, statistics emphasizes on the urgency of developing new chemotherapeutic medicines against cancer cells. Because naturally-occurring compounds show medicinal properties along with low risk of toxicity, they have been proposed to be introduced as chemicals with chemopreventive activity (5). For instance, compounds like polyphenols and resveratrol have been shown to have chemopreventive activities (6, 7). It is almost 3000 years that saffron, stigmas of Crocus sativus is used throughout the world for cooking and medical purposes. While saffron is still more popular for its coloring and flavoring properties, there is considerable evidence that saffron could be considered as a medicine. Crocin, safranal, crocetin (precursor of crocin), and picrocrocin (precursor of safranal) besides being responsible for saffron unique color, odor, color, and taste, respectively, are considered as saffron major pharmacologically active components (8-11). Regarding cancer treatment, it was reported that saffron has selective anticancer properties, both in vitro and in vivo (12, 13).
Crocin is regarded as a carotenoid pigment (Figure 1) (12). As a member of tetraterpene family, carotenoids consist of over 600 known compounds produced exclusively by plants, fungi, bacteria, and algae (14), which are reported to have antimutagenic and anticarcinogenic properties (15). Continuing our studies on cytotoxic properties of crocin on cancerous cell lines (16).
2. Objectives
In this study, we aimed at evaluating the statuses of apoptosis or necrosis profile, reactive oxygen species (ROS) production, DNA fragmentation, and the level of HSP 70 and 90 on U266, B lymphocyte human myeloma cell line, 24 and 48 hours after crocin treatment.
3. Materials and Methods
3.1. Materials
This study was performed using the following items: Annexin V-FITC apoptosis/necrosis detection kit and fluorescent probe 2′,7′-dichlorofluorescein diacetate (DCFH-DA) (Abcam, USA), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium (MTT) and propidium iodide (PI) ( Sigma, USA), RPMI 1640, FBS (fetal bovine serum), penicillin and streptomycin (Gibco, USA), rabbit polyclonal Hsp70 antibody, rabbit polyclonal Hsp90 antibody, anti-rabbit IgG horseradish peroxidase-conjugated antibody, mouse monoclonal β-actin antibody, (Cell signaling, USA), polyvinylidene fluoride (PVDF) membrane (Bio-Rad, USA) and enhanced chemiluminescnces (ECL) reagent for western blot detection (Pierce).
3.2. Extraction, Identification and Purification of Crocin
The stigmas of Crocus sativus (saffron) were collected from Ghaen, Khorasan Razavi, east of Iran and a sample was kept in the Herbarium of the School of Pharmacy, Mashhad University of Medical Sciences. The procedure of crocin identification and purification were carried out as previously reported (17).
3.3. Cell Culture
U266 (C151, B lymphocyte human myeloma) cell line was purchased from Pasteur Institute, Tehran, Iran. Cells were incubated at 37°C, with 90% humidity, and 5% CO2. Cell culture was done in RPMI 1640 medium enriched by 10% fetal bovine serum (heat inactivated), 100 U/mL penicillin and 100 µg/mL streptomycin.
3.4. MTT Assay
Cell viability was evaluated by the colorimetric MTT assay as previously reported (18, 19). In a 96-well plate (4 × 104 cells/well) cells were treated with crocin 50, 250, and 500 µM. After 24 and 48 hour incubation periods, cells were incubated with MTT solution at the concentration of 5 mg/ml for 3 hours at 37°C. Subsequently, medium was aspirated and dimethyl sulfoxide (DMSO) was added to dissolve the fromazan crystals. Finally, the absorbance at 545 nm (reference wavelength: 630 nm) was determined using Stat Fax 2100 ELISA reader (UK).
3.5. Assessment of DNA Fragmentation
Propidium iodide (PI) staining was employed to assess the DNA fragmentation. Based on the method of de Lima et al. (with insignificant adjustments), following the treatment of cells with crocin 50, 250 and 500 µM in a 6-well plate (5 × 105 cells/well) for 24 and 48 hours, cells were washed by PBS and subjected to 15 minutes incubation with RNase A (150 µg/mL) at 37°C (20). Then, cells were incubated with PI solution (100 µg/mL PI, 0.1 % Triton X-100 and 0.1 % sodium citrate) for 30 minutes at 4°C. As the final step, flow cytometric evaluation of fluorescence and data analysis were done by BD FACSCalibur cytometer (Beckton-Dickenson, USA) and CellQuest (USA) software, respectively.
3.6. Determination of ROS Production
Production of ROS in U266 cells following the treatment with crocin was evaluated using 2′, 7′-dichlorofluorescein diacetate (DCFH-DA) kit. After the incubation periods, cells were treated with DCF-DA 20 µM for 30 minutes at 37°C, washed with cold PBS and suspended in PBS. Finally, the florescence intensity of dichlorofluorescein was evaluated by FACSCalibur cytometer (Beckton-Dickenson, USA) and data analysis was done using Cell Quest (USA) software.
3.7. Evaluation of Phosphatidylserine Externalization
To monitor apoptotic events, a commercially available Annexin V-FITC/PI apoptosis detection kit was used. Cells were incubated with Annexin V-FITC (5 µL) and PI (5 µL) for 5 minutes in dark at room temperature. Flow cytometric assessment of 1×104 events were carried out through FL-1 filter (530 nm) and FL-2 filter (585 nm).
3.8. Western Blot Analysis of the Expression of Heat Shock Proteins 70 and 90
In order to extract the proteins, cells were washed twice with ice-cold phosphate-buffered saline (PBS) and lysed as previously described (21), using lysis buffer. Subsequently, separation of the extracted proteins was carried out using 12% SDS polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene fluoride (PVDF) membranes. Next, membranes were blocked by 5% skim milk at room temperature, overnight. Blots were incubated in the presence of antibodies: Hsp70 (cell signaling #4872), and Hsp90 (cell signaling #4874) at the dilution of 1:1000 for 2 hours at room temperature. Blots were washed 3 times in TBST solution (TBS with 0.1% Tween 20) and incubated with horseradish peroxidase-conjugated anti-rabbit antibody (cell signaling #7074) at the dilution of 1:3000 for 1 hour at room temperature and washed 3 times with TBST solution. The analysis of protein bands was performed using UVtec software (UK). Protein levels were normalized against β-actin intensity.
3.9. Statistical Analysis
For each group, results are shown as mean ± SD. By analysis of variance (ANOVA) and Dunnett as the posttest, differences between control and other groups were examined using InStat 3.05 software for Windows and P < 0.05 was considered significant.
4. Results
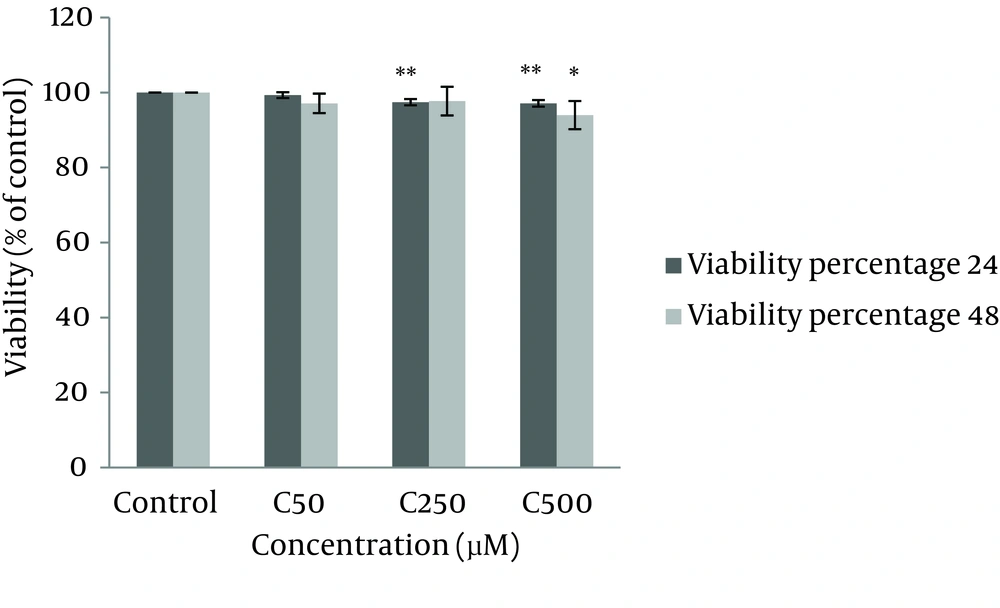
4.1. Cell Viability
According to our results from MTT assay, after 24 hours, crocin (250 and 500 µM) significantly reduced cell viability and proliferation compared with control (P < 0.01). However, after 48 hours incubation only crocin 500µM resulted in a significant decrease in the number of viable cells (Figure 2).
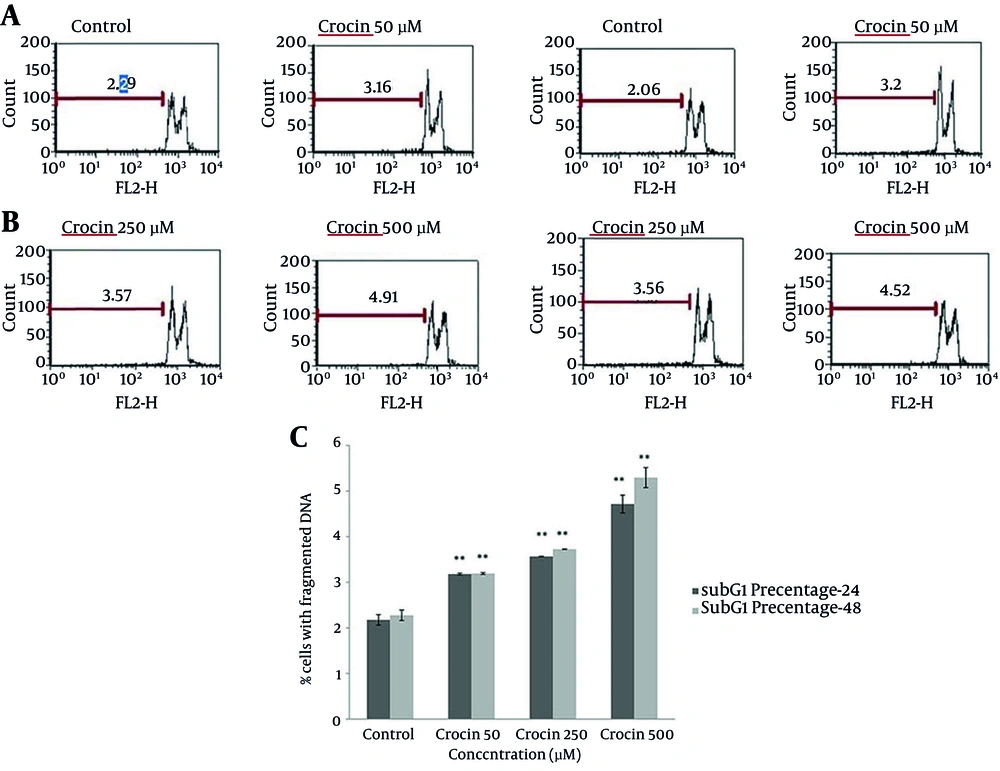
4.2. Assessment of DNA Fragmentation
The population of cells with fragmented DNA were calculated by flow cytometric measurement of PI staining. In comparison with the control group, following both incubation periods, crocin at all doses increased the percentage of cells detected with DNA fragmentation (P < 0.01) (Figure 3).
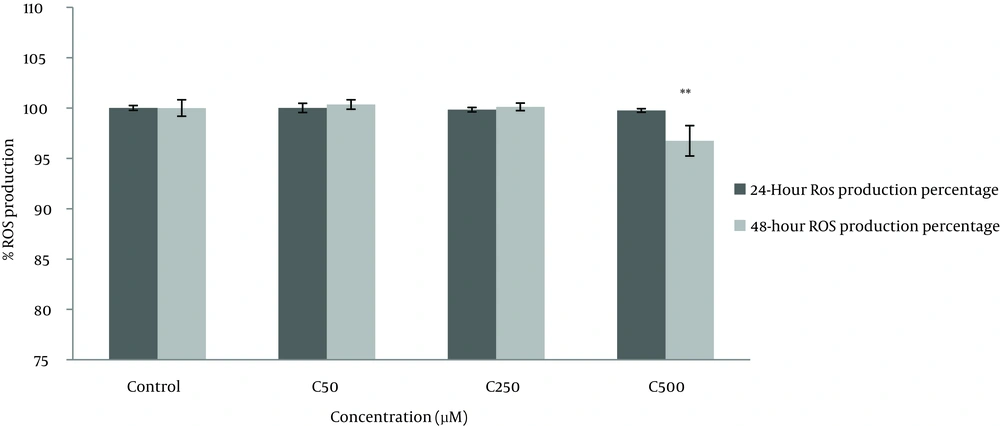
4.3. Determination of Reactive Oxygen Species Production
Generation of ROS was assessed by a commercially available kit. DCFH-DA, a nonpolar and nonfluorescent chemical, freely crosses the cell membrane and is hydrolyzed to the nonfluorescent DCFH by intracellular esterase. In the presence of ROS, DCFH will be oxidized to dichlorofluorescein (DCF) which is greatly fluorescent. As shown in Figure 4, only crocin 500 µM could significantly decrease ROS production after 48 hours of treatment (P < 0.01).
4.4. Evaluation of Phosphatidylserine Externalization
Flow cytometric analysis of phosphatidylserine externalization as the apoptotic surface marker was performed using Annexin V-FITC commercial kit. Our results demonstrated that apoptosis was significantly induced by crocin (250 and 500 µM) following 24 and 48 hours. Treatment with crocin 500 µM for 48 hours resulted in the most profound apoptosis as compared with the control group. However, regarding the necrosis, no significant alterations were recorded (Figure 5).
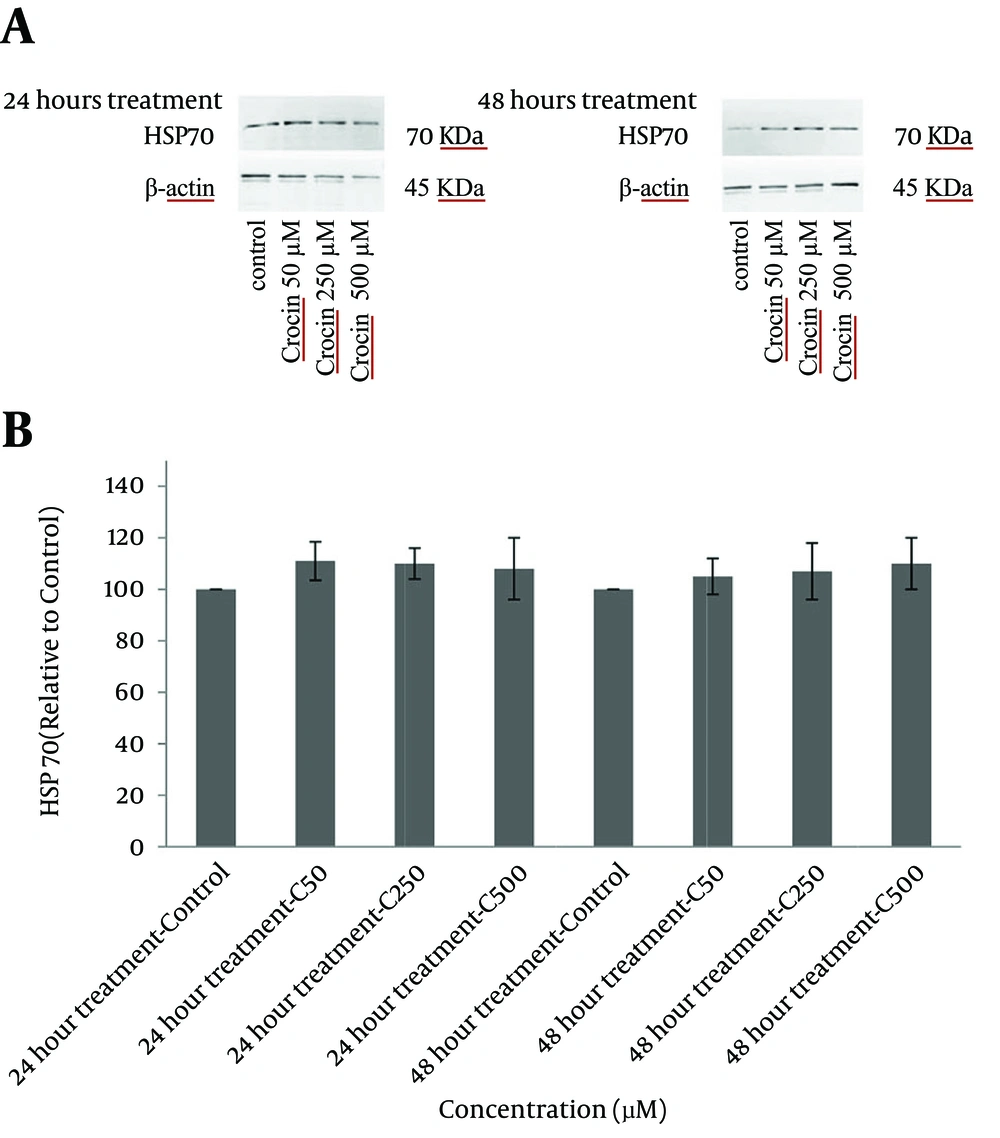
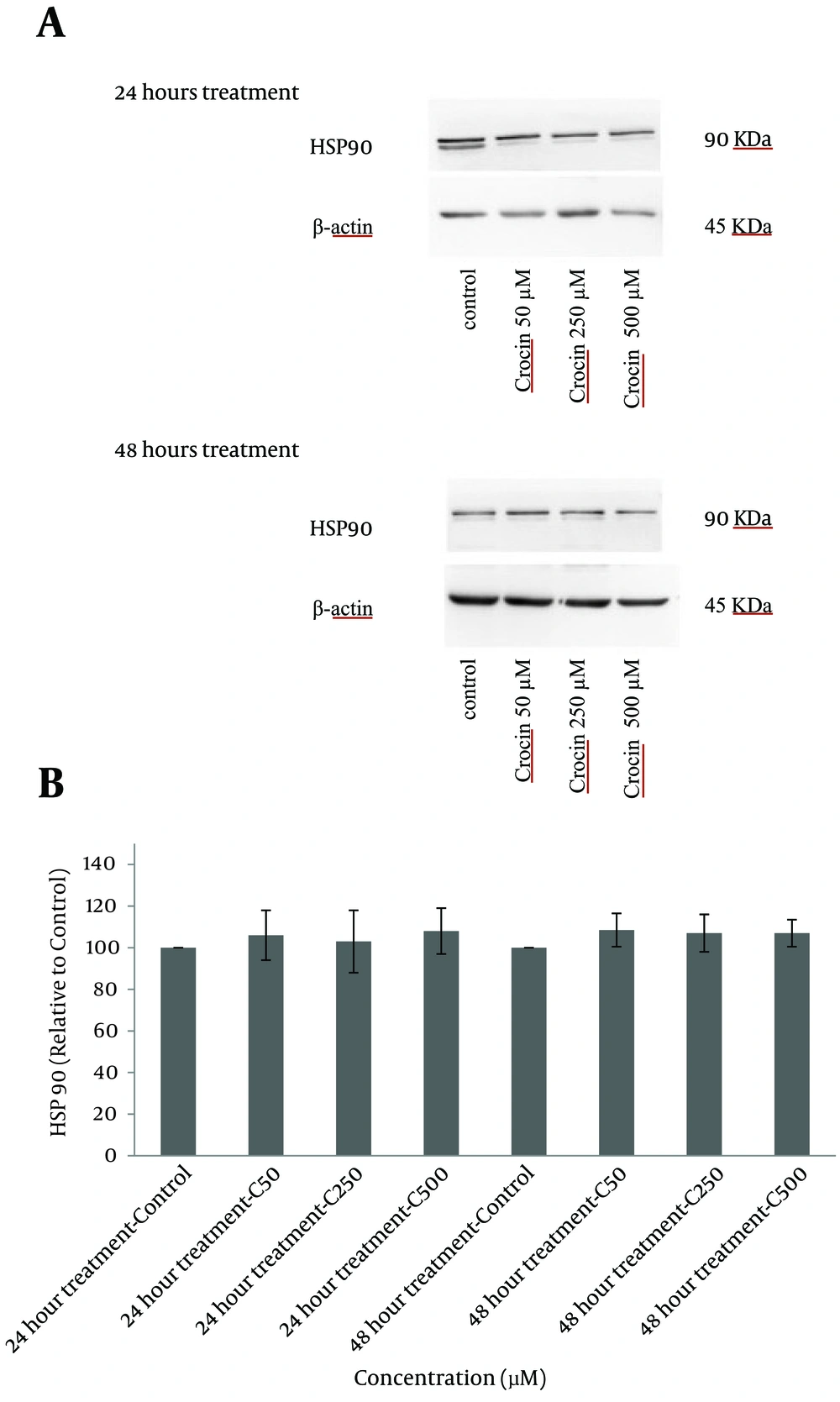
4.5. Western Blot analysis of the Expression of Heat Shock Proteins 70 and 90
The levels of HSP 70 and 90 were determined using western blot analysis. As compared with the control group, treatment of cells with crocin did not change the expression of these proteins considerably and differences between treated groups and control were insignificant after both incubation times (Figures 6 and 7).
A, western blots demonstrating specific bands for HSP 90 and β-actin as an internal control. Equal amounts of protein sample (50 μg) obtained from the cell lysate were applied in each lane. Western blot analysis was carried out for four separate experiments; B, data are expressed as mean ± SD and differences between treatment groups and control are analyzed statistically.
A, western blots demonstrating specific bands for HSP 90 and β-actin as an internal control. Equal amounts of protein sample (50 μg) obtained from the cell lysate were applied in each lane. Western blot analysis was carried out for four separate experiments; B, data are expressed as mean ± SD and differences between treatment groups and control are analyzed statistically.
5. Discussion
Proteomic findings have shown that crocin can attach to a broad range of cellular macromolecules such as structural proteins, membrane transporters, and enzymes involved in redox homeostasis and signal transduction. β-Actin-like protein 2 has been recognized as one of the target proteins of crocin. Actin filaments maintain cell morphology and mediate functions such as adhesion, motility, exocytosis, endocytosis and cell division (22). According to the mentioned mechanism, crocin might have destructive or useful effects on cells. Based on the fact that apoptosis plays an important role in the life span of normal and cancer cells, agents that are able to alter apoptosis rate could possibly influence the steady-state cell populations (23). Thus, in the present study crocin effects on U266 cell line were evaluated regarding cell death pattern, production of reactive oxygen species, DNA fragmentation and the expression of HSP 70 and 90 proteins.
Apoptotic activity of crocin (1, 2, and 4 mM) and crocin liposomal form (0.5 and 1 mM) on HeLa and MCF-7 cells was evaluated. As compared to non-malignant cell line (L929), crocin caused notable cytotoxic effects on HeLa and MCF-7 when the encapsulated form of crocin was used. Moreover, flow cytometric examination showed a sub-G1 peak proposing the role of apoptosis in crocin cytotoxicity (24). Hoshyar et al. reported that although crocin is able to augment the sub-G1 population, caspases activity and Bax/Bcl-2 ratio in the gastric adenocarcinoma (AGS) cells, these factors remained at the normal level in human normal fibroblast skin cells (HFSF-PI3) (25). Xu et al. demonstrated that the proliferation of HL-60 cells was efficiently inhibited by crocin and the number of cells in G₀/G₁ phase was increased. Authors proposed that inhibition of Bcl-2 and activation of Bax might be the main causes of apoptosis induction in HL-60 cells (26). Based on our data, crocin reduced cell viability and proliferation at higher doses at which an increase in apoptosis rate is noticeable. It seems that an increase in the apoptotic cell percentage might be due to the amplification of DNA fragmentation as assessed by PI staining. Crocin at the highest dose led to a significant decline in ROS generation, which is justified by its antioxidant properties. This activity has been reported previously (27).
Three main families of heat shock proteins (HSPs) are high molecular weight HSPs such as HSP90, HSP70, and small HSPs (28). While these proteins are expressed at a low level under normal physiological conditions, a variety of environmental stresses could augment their expression (29). In our study, the levels of HSP 70 and 90 were not altered following crocin treatment as compared with the negative control group. It has been reported that when the cell death takes place in a short period of time, the time for HSPs to rearrange to their effective form or to be delivered to the site of injury is short. Besides, sampling time, intensity of cell damage and the agent which is responsible for the stress are determining factors in the expression level of HSPs. Our western blot analysis results showed no significant alterations in comparison with the control group that might be due to variation of these parameters (30-32). Altogether, crocin showed little cytotoxic effects on U266 cell line and increased the apoptosis because of DNA fragmentation. Because in our study, no prominent effect (even at high concentrations of crocin) was seen, in order to have a better perspective of its activity against cancerous cell lines, further studies are highly recommended.
![Evaluation of Apoptosis or Necrosis of U266 Cells Following 24 and 48-hour Treatment with crocin Examined by Annexin V/PI Test (early Apoptotic cells Were Annexin V [+] and PI [-], Late Apoptotic cells were Annexin V [+] and PI [+], Necrotic Cells Were Annexin V [-] and PI [+] and the Living cells Annexin V [-] and PI [-]) A and B flow cytometry histograms of treated groups and control after 24 and 48 hours, respectively. C and D bar charts show apoptosis and necrosis as mean ± SD of three separate experiments, respectively. (* and **represent P < 0.05 and P < 0.01, respectively).](https://services.brieflands.com/cdn/serve/3170b/436015b85a9d2ef1a94fd40212ceca2bff09fa3a/jjnpp-09-04-20131-i004-preview.webp)