Abstract
Background:
Pulmonary fibrosis is a deadly and destructive disease. Various chemicals, drugs, and environmental factors are responsible for pulmonary fibrosis. The disease can also be idiopathic. A number of inflammatory mediators and cytokines, such as interleukin-13 (IL-13), platelet‐derived growth factor (PDGF), and tumor necrosis factor α (TNF-α), play an important role in pulmonary fibrosis. Profibrotic cytokines accelerate the maturation and proliferation of fibroblasts, which ultimately increases the amount of collagen deposits in the alveolar cell wall. In contrast, the interferon-γ (INF-γ) cytokine has antifibrotic properties.Objectives:
This study investigated and compared the inhibitory effects of quercetin hydrate with those of vitamin E (Vit E), a well-known antioxidant, on bleomycin (BLM)-induced pulmonary fibrosis in rats.Materials and Methods:
This study used Sprague-Dawley male rats, with a weight range of 200 - 250 g. The positive control group received a single-dose of BLM (7.5 IU/kg) through an intratracheal injection (positive control group). The negative control group received normal saline. The other groups were treated with different doses of quercetin hydrate (25, 50, and 75 mg/kg) or daily intraperitoneal injections of Vit E (10 mg/kg) for 1 week before and 3 weeks after a single dose of BLM via intratracheal administration. Four weeks later, bronchoalveolar lavage fluid (BALF) was collected for measurement of cytokines.Results:
The administration of quercetin hydrate significantly reduced the concentration of IL-13, PDGF-β, and TNF-α and increased the concentrations of INF-γ in BALF.Conclusions:
According to the experimental results, quercetin hydrate reduced the concentration of inflammatory fibrotic factors, such as IL-13, PDGF-β, and TNF-α, and increased the level of the antifibrotic factor INF-γ, and the effects of quercetin hydrate appeared stronger than those of Vit E. In cases of exposure to fibrogenic factors, quercetin hydrate can be prescribed as a prophylactic agent for pulmonary fibrosis.Keywords
Pulmonary Fibrosis Cytokine Quercetin Hydrates rat Vitamin E
1. Background
Quercetin, a plant flavonoid, has the highest antioxidant capacity of all the flavonoids and is about six times more potent than vitamin C (1). Flavonoids, which are found in many plants and vegetables, especially onions and broccoli, as well as in green tea, apples, and grapes, have anti-inflammatory, anticoagulant, antibacterial, and antihypertensive effects (2-5). Numerous epidemiological studies have demonstrated a relationship between the consumption of quercetin by enhance the treatment of cardiovascular diseases, lung cancer, asthma, and other respiratory disorders (6). Despite a large number of studies in recent years, there is not any standard and perfect treatment for pulmonary fibrosis. In addition, an ideal drug or combination of drugs is not available (7, 8).
Pulmonary fibrosis is an interstitial lung disease of the respiratory alveolar system (9). In general, it commences with damage to the air bags and can be accompanied by inflammation, collagen accumulation, and accumulation of extracellular matrix components (9). Pulmonary fibrosis results in the stimulation of endothelial and epithelial cells, followed by the release of free radicals and the secretion of a variety of cytokines. By stimulating fibroblast proliferation in the interstitial area of air sacs, these factors cause deposition of collagen on the surface of the wall of the alveolus (10).
In contrast to pulmonary fibrosis, where the cause of the disease is clear, there is no known cause of the disease in idiopathic pulmonary fibrosis (11). Pulmonary fibrosis disease has been called an industrial society disease because most of its causes are the result of the side effects of industrialization. Among the known causes of the disease are viral infections caused by some herpes viruses and bacteria, injury resulting from some mineral compositions like asbestos and silica, and side effects of chemical drugs, such as bleomycin (BLM), cyclophosphamide, parquat, and methotrexate (12). BLM is an anticancer antibiotic drug that has abundant applications for chemotherapy of the cervical testicular cancer (13). Its potency and lack of adverse effects make BLM ideal for the treatment of these kinds of cancers. However, pulmonary fibrosis is a common side effect of BLM (13, 14). Although the mechanisms underlying the pulmonary pathology of BLM are not well known, the drug is thought to chelate various elements, such as iron and copper, which bind to oxygen molecules. The resulting complex binds to DNA molecules and by transferring electrons from iron II to oxygen molecules produces free radical spices, like hydroxyl and super oxide. These radicals cause cleavage of DNA, eventually leading to the death of cells (15). The accumulation of inflammatory cells in lung tissue and collagen deposition in the alveolar wall are additional adverse effects of BLM.
Fibrotic factors, such as tumor necrosis factor-α (TNF-α), platelet‐derived growth factor (PDGF), and interleukin-13 (IL-13), as well as antifibrotic factors, such as interferon-γ (INF-γ), play a very important role in the occurrence and prevention of pulmonary fibrosis (16). PDGF, which is produced by macrophages, causes the growth and proliferation of fibroblasts, thereby exacerbating pulmonary fibrosis. The inhibition of PDGF is one of the goals of fibrosis treatment (17). IL-13 is an inflammatory factor that results in the proliferation of myofibroblasts and the production of collagen (18). It can lead to the production of tumor growth factor-β (TGF-β), which is strong fibrotic factor (18). TNF-α is one of the strong and well-known factors that by IL-13 gene expression lead to the proliferation of fibroblasts and production of collagen and fibrosis (19). INF-γ has extremely strong antifibrotic properties (20). It inhibits the activity of profibrotic factors, such as TGF-β, reducing the growth and differentiation of fibroblasts and collagen synthesis (20).
In previous studies, the therapeutic approach of this disease was in two directions: first, the use of antioxidant compounds, chemical, or plant, to remove free radicals induced and prevent the process of inflammation and second the use of antifibrotic compounds for the treatment and removal of collagen deposited in the alveolar wall. In most previous studies, the use of antioxidant compounds, such as bilirubin (21), N-acetyl cysteine (22), vitamin E (Vit E) (23), Ginkgo bilobia (24) and some compounds of plant considered.
2. Objectives
According to studies in the field of the use of antioxidant compounds for the prevention and treatment of pulmonary fibrosis in rats were investigated. This study aimed to compare the effects of quercetin hydrate and Vit E on levels of IL-13, INF-γ, TNF-α, and PDGF in BLM-induced pulmonary fibrosis in rats.
3. Materials and Methods
3.1. Animals
Sixty healthy male Sprague-Dawley rats weighing 200 - 250 g were obtained from the animal house and research center of Ahvaz Jundishapur University of Medical Sciences (AJUMS), Ahvaz, Iran. The animals had free access to standard rat chow and tap water ad libitum. They were kept in a temperature-controlled environment at 23 ± 2°C, with an alternating 12 hours light: 12 hours dark cycle. The animal experimental protocol was approved by AJUMS Animal care and use committee (25).
3.2. Chemicals
The chemical materials used in this study were as follows: Bleomycin sulfate (Bleocip), was obtained from the chemical company CIPLA (India), quercetin hydrate was obtained from Sigma (St. Louis, Mo, Germany), and ELISA kits for TNF-α, INF-γ, PDGF-β, and IL-13 were purchased from bioassay technology (China).
3.3. Classification and Animal Treatments
The animals were randomly divided into six groups of 10 rats. The composition of the groups was as follows:
Saline group: The animals received equal amounts of saline every day during the experimental period.
BLM group: The animals received a single intratracheal instillation of BLM (7.5 IU/kg) dissolved in normal saline and monitored for 3 weeks after the administration of BLM.
BLM + Q25 group: The animals were treated with quercetin hydrate at a dose of 25 mg/kg (Q25) for 7 days before and 3 weeks after a single dose of BLM (7.5 IU/kg).
BLM + Q50 group: The animals were treated with quercetin hydrate at a dose of 50 mg/kg (Q50) for 7 days before and 3 weeks after instillation of a single dose of BLM (7.5 IU/kg )
BLM group + Q75: The animals were treated with quercetin hydrate at a dose of 75 mg/kg (Q75) for 7 days before and 3 weeks after instillation of a single dose of BLM (7.5 IU/kg).
BLM group + Vit E: The animals were treated with Vit E at a dose of 10 mg/kg (IP) for 7 days before and 3 weeks after instillation of a single dose of BLM (7.5 IU/kg)
3.4. BLM-Induced Pulmonary Fibrosis
According to the method of Schraufnagel et al. (26), the rats were anesthetized with ether, placed on a slanted board, and hanged from their upper incisors. Keeping the nose of the animal closed and its tongue out, the BLM solution (7.5 IU/kg) was delivered via the mouth into the trachea by a modified needle in a volume of 1 mL/kg animal body weight. Control rats received intratracheal instillations of the same volume of saline. After recovery from anesthesia, the rats were returned to their cages.
3.5. Collection of Bronchoalveolar Lavage Fluid (BALF)
BALF was obtained by washing the right lung three times with a total of 1.5 ml of saline through a tracheal cannula (27). The BALF was then centrifuged immediately at 1100 rpm for 10 minutes. The supernatants of BALF were stored at -80°C until the time of the cytokine measurement.
3.6. Measurement of the Cytokines
The concentrations of the cytokines in the BALF were measured by ELISA kits (Bioassay Technology Company, China) using the direct sandwich ELISA method.
3.7. Statistical Analysis
A one-way analysis of variance (ANOVA) was used for the analysis of the average differences of quantity. In cases of a significant difference between the groups, Tukey’s test was applied (P ≤ 0.05).
4. Results
4.1. INF-γ in BALF
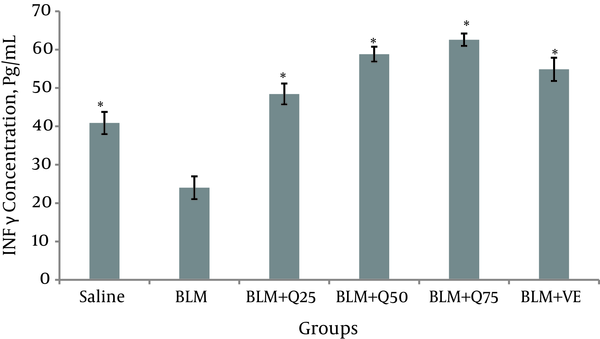
The concentration of INF-γ 21 days after the injection of BLF in the BALF was reduced (24.1 ± 3.4 Pg/mL). The concentrations of INF-γ 21 in the BLM + Q25 mg/kg, BLM + Q50 mg/kg, BLM + Q75 mg/kg, BLM + Vit E 10 mg/kg, and saline group were 48.7 ± 2.9 Pg/mL, 58.4 ± 1.9 Pg/mL, 61.5 ± 1.9 Pg/mL, 55 ± 2.6 Pg/mL, and 40.1 ± 3.4 Pg/mL, respectively (Figure 1).
IFN-γ Level in the BALF of Rats (n = 10)
4.2. IL-13 in BALF
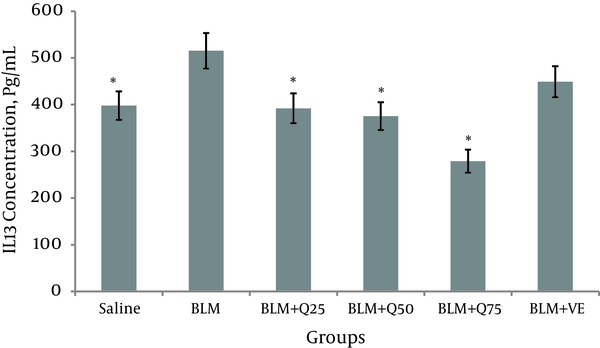
Twenty-one days after the instillation of BLM, the concentration of IL-13 increased significantly in the BALF (515.1 ± 45 Pg/mL) in the saline group and 397.8 ± 3 Pg/mL (P < 0.05). The concentration of IL-13 decreased significantly in the BLM + Q75 mg/kg (279 ± 23 Pg/mL), BLM + Q50 mg/kg (372.4 ± 29 Pg/mL), and BLM + Q25 mg/kg (393 ± 32 Pg/mL) groups compared to the BLM-only group (P < 0.05). However, there was no significant difference in the concentration of IL-13 of the BLM and Vit E groups (448.8 ± 41 Pg/mL), suggesting that Vit E did not reduce the level of IL-13. There was a significant difference between the Q25 mg/kg groups and Vit E group with the Q75 mg/kg group (Figure 2).
Level of IL-13 in the BALF of Rats (n = 10)
4.3. TNF-α in BALF
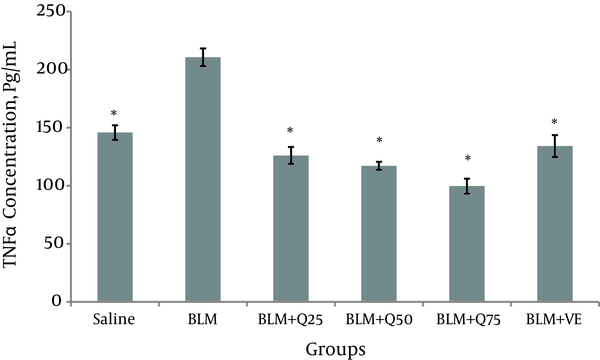
Twenty-one days after the injection of BLM, the concentration of TNF-α in the BALF of the BLM group was (207.7 ± 7 Pg/mL), which was significantly (P < 0.05) higher than that of the saline group (145.8 ± 6.3 Pg/mL). Compared to the BLM group, the concentration of TNF-α decreased significantly in the BLM group and BLM + Q25 mg/kg (126.1 ± 7.3 Pg/mL), BLM + Q50 mg/kg (117.2 ± 3.4 Pg/mL), BLM + Q75 mg/kg (99 ± 6.4 Pg/mL), and Vit E treatment groups (133 ± 14.2 Pg/ml). The results suggest that quercetin hydrate reduced the TNF-α level in the BALF (Figure 3).
The level of TNF-α in the BALF of the rats (n = 10)
4.4. PDGF in BALF
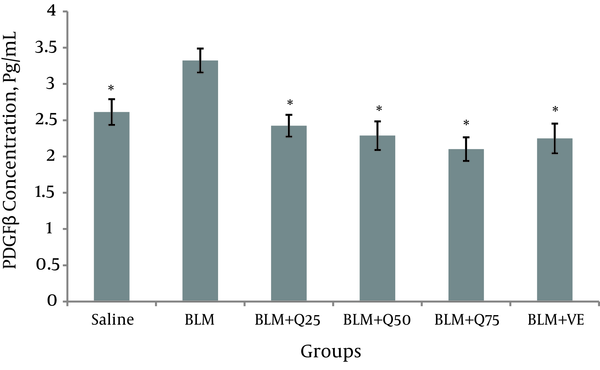
Twenty-one days after the injection of BLM, the concentration of PDGF in the BALF of the BLM group was 3.3 ± 0.26. Compared to the saline group (2.6 ± 0.18), the level increased significantly (P < 0.05). Compared to the BLM group, the level of PDGF was significantly different in the BLM + Q25 mg/kg (2.4 ± 0.21), BLM + Q50 mg/kg (0.21 ± 2.2), and BLM + Q75 mg/kg (2.1 ± 0.17) treatment groups (P < 0.05). As shown in Figure 4, the level of PDGF in the BLM group was also significantly different compared to that of the Vit E group (2.2 ± 0.23).
PDGF Level in the BALF of the Rats (n = 10)
5. Discussion
Pulmonary fibrosis is a complex disease, which begins with edema and inflammation of the alveolar and bronchial walls. An important step in this process is the release of cytokines and growth factors in lung tissue. Further, mesenchymal cells of the alveolar walls are reproduced, and fibroblasts and myofibroblasts move to the alveolar spaces and secrete extracellular matrix compounds, mainly collagen and proteoglycan (28). Such events lead to lung fibrosis. Fibroblasts and myofibroblasts have contractile activity and play an important role in ventilation and perfusion. However, during fibrosis, actin filaments in fibroblast cells are polymerized, and the accumulation of these cells leads to increased contractile power (29, 30). An increase in nonmuscle cells in the interstitial area of pulmonary fibrosis tissue has been established in human samples.
A point that we must take into consideration is the direct effect of the cytokines INF-γ, PDGF, TNF-α, and IL-13 on the growth, differentiation, and maturation of fibroblasts. These cytokines have a very important role in the treatment and diagnosis of pulmonary fibrosis (16, 31). IL-13 is known as one of the dominant cytokines in the majority of experimental studies on pulmonary fibrosis (18), and the treatment of pulmonary fibrosis with anti-IL13 drugs is part of the main strategy for treating and preventing the progression of the disease (32, 33).
In this study, the concentration of IL-13 in the BLM group increased significantly compared with that of the saline group, suggesting that IL-13 was partly responsible for pulmonary fibrosis in the BLM group. In contrast, the level of IL13 decreased significantly in the quercetin treatment groups. This finding demonstrates the potential of quercetin to inhibit the production of IL-13. There was no significant difference between BLM and Vit E, which means the lack of Vit E effect in reducing the IL-13. Perhaps quercetin has priority on Vit E in this field.
TNF-α is one of the most potent cytokines and plays a critical role in inflammatory lung diseases. Several studies have reported a high level of TNF-α in pulmonary fibrosis (34). In this study, the amount of TNF-α in the BLM group was significantly elevated compared to that of the saline group. The administration of quercetin, especially at a dose of 75 mg/kg, significantly reduced the concentration of TNF-α. A significant difference was also seen between the BLM and Vit E groups.
PDGF is a powerful fibrogenic factor, which intensifies the process of pulmonary fibrosis by increasing the growth and proliferation of fibroblasts. The inhibition of PDGF is one of the main goals of fibrosis treatment (17). In this study, the level of PDGF decreased significantly in the quercetin treatment groups compared to the BLM group. The potency of Vit E in reducing the level of PDGF was similar to that of quercetin.
INF-γ exerts antifibrotic effects by inhibiting the activity of profibrotic factors, such as TGF-β. Its effect is mainly due to inhibition of the growth and differentiation of fibroblasts and subsequently a reduction in collagen synthesis (35). In this study, the level of INF-γ decreased significantly following the administration of BLM compared with that of the control group. However, the level of INF-γ increased in all the treatment groups in response to quercetin and Vit E. An important finding in this work is that the elevation in INF-γ was considerably higher in the quercetin-treated groups than in the saline group. Possibly, quercetin may be useful as an immunomodulator in other diseases.
A previous study of the effects of sirolamus in BLM-induced pulmonary fibrosis in rats reported that it decreased the concentration of IL-13 and PDGFT PGF-β and significantly increased the concentration of INF-γ, demonstrating that it had antifibrotic and anti-inflammatory effects (36). Another study of the effect of lycopene on pulmonary fibrosis found that it reduced the concentration of TNF-α, NO, and MDA, establishing that lycopene had antifibrotic effects (37). A study of the influence of simvastatin on BLM-induced pulmonary fibrosis in rats showed that it reduced the concentration of IL-13, PDGF, and TGF-β and increased the concentration of INF-γ (38).
Quercetin hydrate is a polyphenolic compound found in plants and one of the best known of the flavonoids, with the strongest antioxidant effect (39, 40). Quercetin has a wide range of activities within the cell, including interfering with the functioning of enzymes and cell receptors (19). The mechanism of its effect varies in different physiological processes. Several studies have investigated the effect of quercetin and its derivatives on a variety of factors affecting pulmonary fibrosis (41). One study indicated that quercetin significantly decreased the levels of cytokines, such as TNF-α, IL-6, and IL-1-β (42). In addition, this compound lowered hydroxyproline and caused a reduction in collagen deposition in tissue by preventing TGF-β gene expression (42). The administration of quercetin also prevented the progression of pulmonary damage caused by the consumption of paraquat, likely due to its antioxidant properties (43).
In the present study, all three doses of quercetin hydrate reduced the concentration of the fibrotic factors IL-13, TNF-α, and PDGF. Furthermore, its potency was comparable to that of Vit E. On the other hand, both quercetin hydrate and Vit E increased the concentration of the antifibrotic factor INF-γ. The antioxidant properties of quercetin hydrate are greater than those of Vit E. The use of higher doses of quercetin hydrate and longer durations of the treatment may yield better results.
The results of this study showed that the therapeutic profile of quercetin hydrate with regard to the inhibition of pulmonary fibrosis was superior to that of Vit E. Herbal source of quercetin with negligible adverse effects is an opportunity to make the preparations containing this compound. Using quercetin-derived preparations as a complementary treatment is recommended in patients with lung fibrosis. However, more studies may required to determine the detailed mechanism underlying the effects of quercetin on pulmonary fibrosis.
Acknowledgements
References
-
1.
Arts MJ, Sebastiaan Dallinga J, Haenen GR. A new approach to assess the total antioxidant capacity using the TEAC assay. Food Chem. 2004;88(4):567-70. https://doi.org/10.1016/j.foodchem.2004.02.008.
-
2.
Wattel A, Kamel S, Prouillet C, Petit JP, Lorget F, Offord E, et al. Flavonoid quercetin decreases osteoclastic differentiation induced by RANKL via a mechanism involving NF kappa B and AP-1. J Cell Biochem. 2004;92(2):285-95. [PubMed ID: 15108355]. https://doi.org/10.1002/jcb.20071.
-
3.
Kim YJ, Bae YC, Suh KT, Jung JS. Quercetin, a flavonoid, inhibits proliferation and increases osteogenic differentiation in human adipose stromal cells. Biochem Pharmacol. 2006;72(10):1268-78. [PubMed ID: 16996034]. https://doi.org/10.1016/j.bcp.2006.08.021.
-
4.
Vieira EK, Bona S, Di Naso FC, Porawski M, Tieppo J, Marroni NP. Quercetin treatment ameliorates systemic oxidative stress in cirrhotic rats. ISRN Gastroenterol. 2011;2011:604071. [PubMed ID: 21991520]. https://doi.org/10.5402/2011/604071.
-
5.
Chen X. Protective effects of quercetin on liver injury induced by ethanol. Pharmacogn Mag. 2010;6(22):135-41. [PubMed ID: 20668581]. https://doi.org/10.4103/0973-1296.62900.
-
6.
Knekt P, Kumpulainen J, Jarvinen R, Rissanen H, Heliovaara M, Reunanen A, et al. Flavonoid intake and risk of chronic diseases. Am J Clin Nutr. 2002;76(3):560-8. [PubMed ID: 12198000].
-
7.
Hemmati AA, Rezaie A, Darabpour P. Preventive effects of pomegranate seed extract on bleomycin-induced pulmonary fibrosis in rat. Jundishapur J Nat Pharm Prod. 2013;8(2):76-80. [PubMed ID: 24624192].
-
8.
Richeldi L, Davies HRHR, Spagnolo P, Luppi F, Richeldi L. Corticosteroids for idiopathic pulmonary fibrosis. 2003. https://doi.org/10.1002/14651858.cd002880.
-
9.
Chen F, Gong L, Zhang L, Wang H, Qi X, Wu X, et al. Short courses of low dose dexamethasone delay bleomycin-induced lung fibrosis in rats. Eur J Pharmacol. 2006;536(3):287-95. [PubMed ID: 16581064]. https://doi.org/10.1016/j.ejphar.2006.03.011.
-
10.
Demedts M, Costabel U. ATS/ERS international multidisciplinary consensus classification of the idiopathic interstitial pneumonias. Eur Respir J. 2002;19(5):794-6. [PubMed ID: 12030715].
-
11.
Kim DS, Collard HR, King TJ. Classification and natural history of the idiopathic interstitial pneumonias. Proc Am Thorac Soc. 2006;3(4):285-92. [PubMed ID: 16738191]. https://doi.org/10.1513/pats.200601-005TK.
-
12.
Kuwano K, Kunitake R, Maeyama T, Hagimoto N, Kawasaki M, Matsuba T, et al. Attenuation of bleomycin-induced pneumopathy in mice by a caspase inhibitor. Am J Physiol Lung Cell Mol Physiol. 2001;280(2):L316-25. [PubMed ID: 11159011].
-
13.
Adamson IY. Pulmonary toxicity of bleomycin. Environ Health Perspect. 1976;16:119-26. [PubMed ID: 65280].
-
14.
Umezawa H, Ishizuka M, Maeda K, Takeuchi T. Studies on bleomycin. Cancer. 1967;20(5):891-5.
-
15.
Sleijfer S. Bleomycin-induced pneumonitis. CHEST J. 2001;120(2):617-24.
-
16.
Border WA, Noble NA. Transforming growth factor beta in tissue fibrosis. N Engl J Med. 1994;331(19):1286-92. [PubMed ID: 7935686]. https://doi.org/10.1056/NEJM199411103311907.
-
17.
Abdollahi A, Li M, Ping G, Plathow C, Domhan S, Kiessling F, et al. Inhibition of platelet-derived growth factor signaling attenuates pulmonary fibrosis. J Exp Med. 2005;201(6):925-35. [PubMed ID: 15781583]. https://doi.org/10.1084/jem.20041393.
-
18.
Aliprantis AO, Wang J, Fathman JW, Lemaire R, Dorfman DM, Lafyatis R, et al. Transcription factor T-bet regulates skin sclerosis through its function in innate immunity and via IL-13. Proc Natl Acad Sci U S A. 2007;104(8):2827-30. [PubMed ID: 17307869]. https://doi.org/10.1073/pnas.0700021104.
-
19.
Hasegawa M, Fujimoto M, Kikuchi K, Takehara K. Elevated serum tumor necrosis factor-alpha levels in patients with systemic sclerosis: association with pulmonary fibrosis. J Rheumatol. 1997;24(4):663-5. [PubMed ID: 9101498].
-
20.
Moutsatsou P. The spectrum of phytoestrogens in nature: our knowledge is expanding. Hormones (Athens). 2007;6(3):173-93. [PubMed ID: 17724002].
-
21.
Cortijo J, Cerda-Nicolas M, Serrano A, Bioque G, Estrela JM, Santangelo F, et al. Attenuation by oral N-acetylcysteine of bleomycin-induced lung injury in rats. Eur Respir J. 2001;17(6):1228-35. [PubMed ID: 11491169].
-
22.
Wang HD, Yamaya M, Okinaga S, Jia YX, Kamanaka M, Takahashi H, et al. Bilirubin ameliorates bleomycin-induced pulmonary fibrosis in rats. Am J Respir Crit Care Med. 2002;165(3):406-11. [PubMed ID: 11818329]. https://doi.org/10.1164/ajrccm.165.3.2003149.
-
23.
Kilinc C, Ozcan O, Karaoz E, Sunguroglu K, Kutluay T, Karaca L. Vitamin E reduces bleomycin-induced lung fibrosis in mice: biochemical and morphological studies. J Basic Clin Physiol Pharmacol. 1993;4(3):249-69. [PubMed ID: 8679519].
-
24.
Daba MH, Abdel-Aziz AA, Moustafa AM, Al-Majed AA, Al-Shabanah OA, El-Kashef HA. Effects of L-carnitine and ginkgo biloba extract (EG b 761) in experimental bleomycin-induced lung fibrosis. Pharmacol Res. 2002;45(6):461-7. [PubMed ID: 12162946].
-
25.
Larki-Harchegani A, Hemmati AA, Arzi A, Ghafurian-Boroojerdnia M, Shabib S, Zadkarami MR, et al. Evaluation of the Effects of Caffeic Acid Phenethyl Ester on Prostaglandin E2 and Two Key Cytokines Involved in Bleomycin-induced Pulmonary Fibrosis. Iran J Basic Med Sci. 2013;16(7):850-7. [PubMed ID: 23997916].
-
26.
Schraufnagel DE, Mehta D, Harshbarger R, Treviranus K, Wang NS. Capillary remodeling in bleomycin-induced pulmonary fibrosis. Am J Pathol. 1986;125(1):97-106. [PubMed ID: 2430459].
-
27.
Giri SN, Hyde DM, Nakashima JM. Analysis of bronchoalveolar lavage fluid from bleomycin-induced pulmonary fibrosis in hamsters. Toxicol Pathol. 1986;14(2):149-57. [PubMed ID: 2429361].
-
28.
Fell CD, Martinez FJ, Liu LX, Murray S, Han MK, Kazerooni EA, et al. Clinical predictors of a diagnosis of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2010;181(8):832-7. [PubMed ID: 20056903]. https://doi.org/10.1164/rccm.200906-0959OC.
-
29.
Vyalov SL, Gabbiani G, Kapanci Y. Rat alveolar myofibroblasts acquire alpha-smooth muscle actin expression during bleomycin-induced pulmonary fibrosis. Am J Pathol. 1993;143(6):1754-65. [PubMed ID: 7504890].
-
30.
Costabel U, King TE. International consensus statement on idiopathic pulmonary fibrosis. Eur Respir J. 2001;17(2):163-7. [PubMed ID: 11334114].
-
31.
Gogali A, Wells AU. New pharmacological strategies for the treatment of pulmonary fibrosis. Ther Adv Respir Dis. 2010;4(6):353-66. [PubMed ID: 20864457]. https://doi.org/10.1177/1753465810379454.
-
32.
Chiaramonte MG, Cheever AW, Malley JD, Donaldson DD, Wynn TA. Studies of murine schistosomiasis reveal interleukin-13 blockade as a treatment for established and progressive liver fibrosis. Hepatology. 2001;34(2):273-82. [PubMed ID: 11481612]. https://doi.org/10.1053/jhep.2001.26376.
-
33.
Zhu Z, Homer RJ, Wang Z, Chen Q, Geba GP, Wang J, et al. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J Clin Invest. 1999;103(6):779-88. [PubMed ID: 10079098]. https://doi.org/10.1172/JCI5909.
-
34.
Raghu G, Brown KK, Costabel U, Cottin V, du Bois RM, Lasky JA, et al. Treatment of idiopathic pulmonary fibrosis with etanercept: an exploratory, placebo-controlled trial. Am J Respir Crit Care Med. 2008;178(9):948-55. [PubMed ID: 18669816]. https://doi.org/10.1164/rccm.200709-1446OC.
-
35.
Ziesche R, Hofbauer E, Wittmann K, Petkov V, Block LH. A preliminary study of long-term treatment with interferon gamma-1b and low-dose prednisolone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 1999;341(17):1264-9. [PubMed ID: 10528036]. https://doi.org/10.1056/NEJM199910213411703.
-
36.
Tulek B, Kiyan E, Toy H, Kiyici A, Narin C, Suerdem M. Anti-inflammatory and anti-fibrotic effects of sirolimus on bleomycin-induced pulmonary fibrosis in rats. Clin Invest Med. 2011;34(6):E341. [PubMed ID: 22129924].
-
37.
Zhou C, Han W, Zhang P, Cai M, Wei D, Zhang C. Lycopene from tomatoes partially alleviates the bleomycin-induced experimental pulmonary fibrosis in rats. Nutr Res. 2008;28(2):122-30. [PubMed ID: 19083398]. https://doi.org/10.1016/j.nutres.2007.12.008.
-
38.
Tulek B, Kiyan E, Kiyici A, Toy H, Bariskaner H, Suerdem M. Effects of simvastatin on bleomycin-induced pulmonary fibrosis in female rats. Biol Res. 2012;45(4):345-50. [PubMed ID: 23558989]. https://doi.org/10.4067/S0716-97602012000400003.
-
39.
Bjeldanes LF, Chang GW. Mutagenic activity of quercetin and related compounds. Science. 1977;197(4303):577-8. [PubMed ID: 327550].
-
40.
Boots AW, Haenen GR, Bast A. Health effects of quercetin: from antioxidant to nutraceutical. Eur J Pharmacol. 2008;585(2-3):325-37. [PubMed ID: 18417116]. https://doi.org/10.1016/j.ejphar.2008.03.008.
-
41.
Haenen GR, Bast A. Nitric oxide radical scavenging of flavonoids. Methods Enzymol. 1999;301:490-503. [PubMed ID: 9919597].
-
42.
Baowen Q, Yulin Z, Xin W, Wenjing X, Hao Z, Zhizhi C, et al. A further investigation concerning correlation between anti-fibrotic effect of liposomal quercetin and inflammatory cytokines in pulmonary fibrosis. Eur J Pharmacol. 2010;642(1-3):134-9. [PubMed ID: 20510684]. https://doi.org/10.1016/j.ejphar.2010.05.019.
-
43.
Park HK, Kim SJ, Kwon do Y, Park JH, Kim YC. Protective effect of quercetin against paraquat-induced lung injury in rats. Life Sci. 2010;87(5-6):181-6. [PubMed ID: 20600150]. https://doi.org/10.1016/j.lfs.2010.06.011.
