Abstract
Background:
Hydatidosis caused by Echinococcus granulosusus remains an important parasitic disease, mainly in the Mediterranean region, in human and veterinary medicine. Many protoscolicidal agents have been used to treat it, but most of them are not safe and effective due to their undesirable side effects.Objectives:
The aim of the present work was to evaluate the in vitro efficacy of the antihelminths artemether, artemisinine, albendazole, and drug combinations against Echinococcus granulosusus protoscoleces.Materials and Methods:
Echinococcus granulosus protoscoleces were aseptically removed from liver hydatid cysts in sheep. Drugs were used at the following final concentrations: 1, 2.5, 5, 10, 25, 50, 100, and 200 μg/mL for 15 days. The viability of protoscoleces was confirmed by Eosine 0.1%.Results:
In this case, the protoscolicidal effect of artemether and its combinations was significantly (P < 0.05) higher than other groups; the maximum protoscolicidal effect was found with 200 μg/mL of artemether after 4 days whereas albendazole killed protoscoleces on the 7th day post-incubation. Surprisingly, the incubation of protoscoleces with artemisinine exhibited promising results, as only artemisinine was more effective against evaginated protoscoleces on the 9th day. The maximum effect of two drugs combined belonged to artemether and artemisinine.Conclusions:
The obtained outcome demonstrated the desirable effect of artemether against Echinococcus granulosus protoscoleces. With regard to artemisinine’s astonishing results, it seems that artemisinine can be a striking drug for tissue and metacestode forms of hydatidosis in human and animal models. However, further investigations into the in vivo experiments are proposed.Keywords
1. Background
Cystic echinococcosis caused by the larval stage of the cestode Echinococcus granulosusus produces a long-lasting and life-threatening infection in humans and animals, which is a serious public health problem and economic concern worldwide (1). It has been estimated that hydatidosis has affected approximately 50 million people worldwide, particularly in sheep-raising areas. If it is diagnosed, the disease can be successfully treated with drugs; if left untreated, it can rupture and lead to serious complications (2). Treatment options for hydatidosis include non-operative and operative methods. Chemotherapy and percutaneous treatment are used as non-operative methods, and conservative and radical procedures are used for operative methods. Chemotherapy has been used preoperatively or postoperatively or both (3).
Before the introduction of benzimidazole anthelmintics, surgery was the only treatment available (4) and is still the most common treatment for hydatidosis (5).
Mebendazole and albendazole were first used in humans in the late 1970s (6-8). Almost 20 - 40% of cases did not respond to chemotherapy (9). Albendazole has been shown to be significantly more effective than mebendazole in the treatment of whole hydatid cysts and liver cysts (10). The estimated effectiveness of benzimidazole treatments, including albendazole, is lower than 80%, with only 8% - 20% of patients appearing to have successful treatment, as indicated by the disappearance or significant decrease in the size of cysts (11).
The earliest report on the use of Artemisia annua was in the Recipes for 52 kinds of diseases found in the Mawangdui Han Dynasty tomb dating from 168 B.C. Later, it was introduced in 1798 as a treatment for malaria (12). Nowadays, artemisinine and its derivatives are considered as the treatment for malaria in Africa by the WHO (13). Artemisinine and its derivatives are effective against multidrug-resistant plasmodium falciparum strains mainly in Southeast Asia and Africa, without any reported cases of resistance (14). Artemisinine and its derivatives have also been shown to be effective against a number of viruses, Pnuemocystis carinii, Toxoplasma gondii, a number of human cancer cell lines (15), and a variety of other parasitic tropical diseases including schistosomiasis (16), leishmaniasis (17, 18), Chagas disease, and African sleeping sickness (19).
Artemether is a semi-synthetic derivative of artemisinine and the most widely used antimalarial drug (20). Artemether is converted primarily to dihydroartemisinine, which is often (90%) bound to plasma proteins (21). It has exhibited a broad spectrum of activity against blood and tissue flukes in vitro and in vivo (22). Shalaby et al. in 2009 showed that artemether can be used as an effective drug against nematode (23).
1.1. Pharmacokinetics
Albendazole is a benzimidazole carbamate with better absorption properties. Its active metabolite against protoscoleces of Echinococcus granulosis is albendazole sulfoxide, and it is able to penetrate into hydatid cysts (24). The mode of action of benzimidazoles is the interaction with the eukaryotic cytoskeletal protein, β-tubulin, inhibiting its polymerization into microtubules, reducing glucose uptake and leading to the depletion of glycogen storage, degenerative alterations in the endoplasmic reticulum and mitochondria of the germinal layer and finally, cellular autolysis (25, 26). The effect of albendazole against metacestodes Spp., such as Echinococcus spp., was investigated in animal models in the 1970s (27, 28). The ability of the drug to penetrate the complex structure of the cyst’s wall, which mainly depends on drug’s lipophilicity, is the most critical factors for the drug’s success (29).
Artemisinine, the parent compound for semisynthetic derivatives, has been chemically modified to produce artemether and other derivatives (21). To Artemisinin and its derivatives are mostly used as antimalarial drugs (20). These compounds have been administrated orally and rectally. The mechanisms of action for artemisinine and artemether are unclear. Specific reactions of artemisinine with translationally controlled tumor protein (TCTP), the inhibition of the sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) orthologue (PfATP6) of P. falciparum, and the inhibition of P. falciparum cysteine proteases have been proposed (30). Artemisinine is rapidly absorbed from the gastrointestinal tract and the peak plasma level occurs at 1 hour and is hydrolyzed in vivo to the active metabolite dihydroartemisinine. Then, the parent compound and metabolite are widely distributed in the tissues and the half-life is 4 hours with no major side effects (31). The peak plasma concentration of artemether occurs in 6 hours with a half-life of 4 - 11 hours (31).
2. Objectives
Given the abovementioned points, the aim of the current experimental work was to demonstrate the efficacy of artemether, artemisinine, albendazole, and drug combinations against Echinococcus granolosus protoscoleces. Considering the limited diversity of protoscolicidal drugs, the development of new protoscolicidal compounds has been of great interest.
3. Materials and Methods
3.1. Drug Preparation
Artemether (Sigma-Aldrich, USA), artemisinine (Sigma-Aldrich, USA), and albendazole (Yabang-QH, China) were dissolved in ethanol and dimethyl sulfoxide (DMSO) as stock solutions of 2000 μg/mL and diluted with sterile phosphate buffer solution (PBS) at serial concentrations of 1, 2.5, 5, 10, 25, 50, 100, and 200 μg/mL (400 μg/mL was used only for artemisinine). Penstrep was purchased from Invitrogen (Gibco®). Artemether and artemisinine were evaluated in comparison to albendazole as the reference drug.
3.2. Protoscoleces Collection
Echinococcus granulosus protoscoleces were aseptically removed from liver hydatid cysts from sheep slaughtered at the municipal abattoirin Tabriz, Iran. The hydatid cysts (1.5 - 6 cm in diameter) were cut open, and hydatid fluid was aseptically transferred into a 50 mL Falcon tube and centrifuged at 1500 rpm for 10 minutes at 37°C. The viability of protoscoleces was assessed microscopically using an Eosin 0.1% (1 g of eosin powder in 1000 mL of distilled water) exclusion test and motility of flame cells. Protoscoleces were washed four times in Hanks’ balanced salt solution (Ph = 7.4) containing 10% glucose and 200 U/mL of penicillin, 200 mg/mL of streptomycin, and 0.5 mg/mL of amphotericin B supplemented to eliminate the remaining hydatid membranes and fluid (32). Protoscoleces were treated with 0.1% pepsin in Hanks’ salt solution, at a pH of 2.0, at 37°C for 30 - 45 minutes, to eliminate the remnants of the germinal layer and dead protoscoleces. Pepsin was removed by four washings with Hanks’ medium. Then the viability of the protoscoleces was evaluated on the basis of the flame cell activity as observed under a light microscope by Eosin 0.1%.
3.3. Macrophage Apoptosis Assay
To evaluate the effects of artemisinin toxicity on uninfected BALB/c mouse macrophages, we used Annexin V-FITC apoptosis detection kit (Roche, Germany). The macrophages (5 × 105 cells/mL) were exposed toartemisinin (5, 10, 25, 50, and 100 µg/mL) in an RPMI 1640 medium (Gibco BRL) supplemented with 10% FBS (Gibco BRL). Cell cultures were incubated at 37°C and 5% CO2. After 48 hours, the samples were collected and centrifuged in 1400 g and 4°C for 10 minutes. Apoptosis assay was done by flow cytometry (33).
3.4. In Vitro Scolicidal Assay
The number of protoscoleces that was 100 in per mL of DMEM was added to DMEM separately in 24-well tissue culture plates, each containing 200 U/mL of penicillin, 200 mg/mL of streptomycin, and 0.5 mg/mL of amphotericin B) supplemented (34). Culture plates were placed in an upright position in an incubator at 37°C and 5% CO2, without medium changes. Drugs were added at 1, 2.5, 10, 25, 50, 100, and 200 μg/mL to protoscoleces and 400 μg/mL was used only for artemisinine. Tests were carried out in duplicate; ethanol and DMSO (drug solvents) with equal volumes without the drugs were used as controls. For the preparation of drug combinations, 1, 2.5, 10, 25, 50, 100, and 200 μg/mL of them were prepared with equal proportions of artemisinine, albendazole, and artemether.The viability of protoscoleces was followed microscopically using an Eosin 0.1% exclusion test. At first 12 hours, assessment was done every hour. Then, assessment was done daily, every 24 hours. Cultures were kept in a culture plate (SPL life science Co. Korea) placed in an incubator at 37°C, 5% CO2 without changes in themedium during the entire incubation period (35). Non-treated protoscoleces were considered as control groups. The number of live protoscoleces was counted daily up to the 15th day. The rate of protoscoleces viability was calculated according to the number of live protoscoleces per field out of 100 protoscoleces seen under the microscope.
3.5. Statistical Analysis
To determine the differences between tests and control groups, statistical analysis was performed using SPSS 16.0, Mann-Whitney U. A P value of less than 0.05 was considered significant.
4. Results
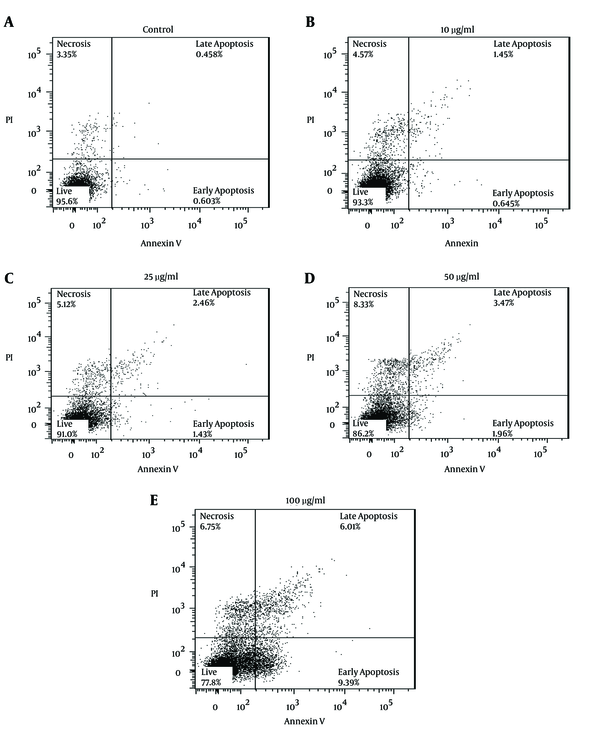
The toxic effect of different concentrations of artemisinin on mice’s uninfected macrophages was evaluated after 48 hours. Flow cytometry assay results have been presented below (Figure 1).
Flow Cytometry Results of the Effect of Artemisinin on the Macrophages of BALB/c Mice Viability
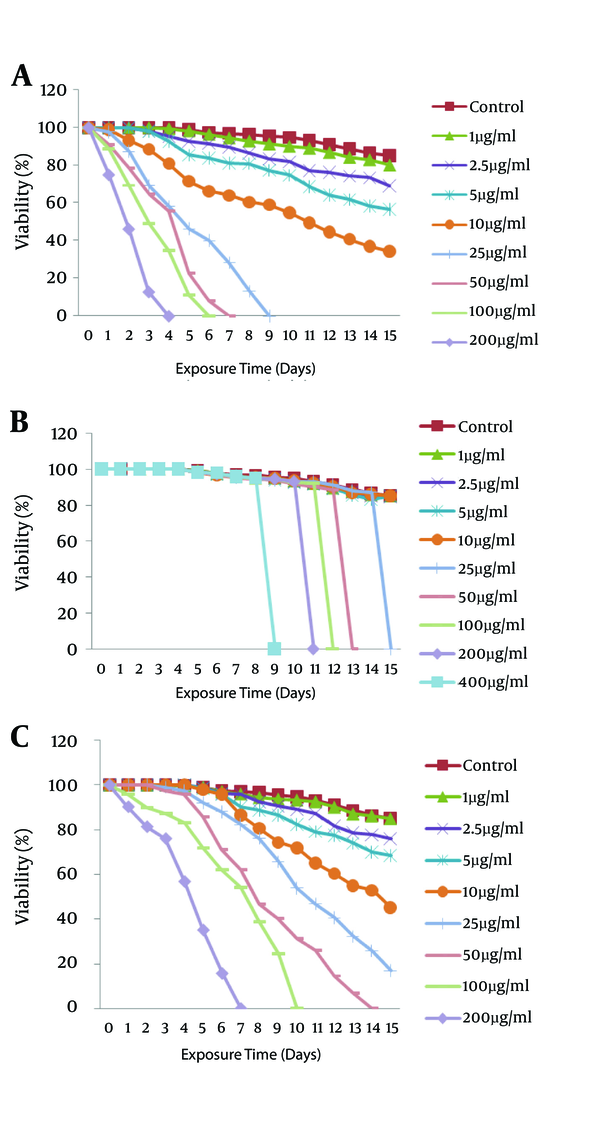
The scolicidal effects of artemether, artemisinine, and albendazole are summarized in Figures 2 - 4. The in vitro treatment yielded different protoscolicidal effects depending on the drugs used, various exposure times, and their combinations. E. granulosus protoscoleces, after exposure to artemether, artemisinine, and albendazole, gradually lost their viability, whereas the viability of the protoscoleces control group was 85% after 15 days of incubation.
Scolicidal effects of A, artemether; B, artemisinine; and C, albendazole to E. granulosus after exposure at different concentrations for 15 days. Each point represents the mean percentage of live protoscoleces.
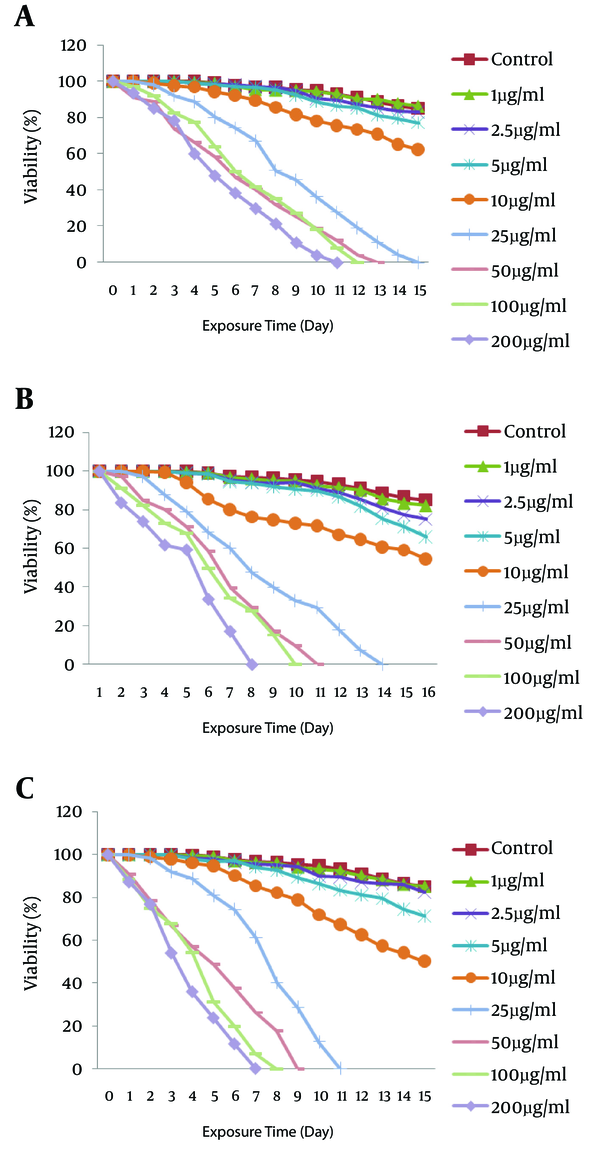
Scolicidal Effects of the Combination of Two Drugs on E. granulosus
Dead Protoscoleces After Exposure to Artemether, Albendazole and Artemisinine
The numbers of viable drug-treated protoscoleces were clearly lower than the control (DMSO- and ethanol-treated) groups; thus, with a concentration of 200 μg/mL of drugs, the viability of protoscoleces was reduced to 0% after 4 days p.i. for artemether (Figure 2A), versus 7 days for albendazole (Figure 2C) and 9 days for artemisinine (Figure 2B). For combined chemotherapy at this time, the mortality of protoscoleces incubated withartemether (100 μg/mL) plus artemisinine (100 μg/mL) was 100% at the 7th day (Figure 3C) versus 11 days for artemisinine (100 μg/mL) plus albedazole (100 μg/mL) (Figure 3A) and 8 days for artemether (100 μg/mL) plus albendazole (100 μg/mL) (Figure 3B). We showed that the reappears to be a synergic effect between artemisinine plus artemether and albendazole plus artemisinine when used in combination.
The scolicidal effect of 200 μg/mL of artemether was extremely significant compared to the same concentrations of artemisinine and its combinations (P < 0.05), although there was no significant difference in comparison to the same concentrations of albendazole, albendazole plus artemether, and artemether plus artemisinine groups (P < 0.05).
The effect of artemisinine against protoscoleces was surprisingly different from other drugs. In spite of artemether and albendazolekilling the protoscoleces gradually over the incubation time (Figure 4A), the protoscoleces exposed to artemisinine were viable by the 8th day of incubation. Surprisingly, the viability rate became 0% on the 9th day (200 μg/mL) for artemisinine as the majority of protoscoleces were evaginated (Figure 4B). This result also was seen for other concentrations of artemisinine.
All experiments exhibited time-dependent and dose-dependent scolicidal effects on the protoscoleces of hydatid cysts. The results of the present study indicated that artemether and artemisinine as new drugs have high scolicidal activity in vitro.
5. Discussion
In this work, we determined that the antimalarial compounds artemether and artemisinine exhibited promising activity against Echinococcus granolosus protoscoleces in vitro. Unfortunately, there are few studies of artemisinine derivatives’ effects against Echinococcus protoscoleces. The present work is the first report of artemether, artemisinine, and albendazole and their combinations against protoscoleces to explore their possible synergic effects. Albendazole was used as the standard treatment against echinococcosis; as the most commonly used drug in echinococcosis treatment, it has no clear treatment time course for pre- and post-operative cases. However, the current prolonged recommended duration of drug therapy, which may last up to 6 months, predisposes patients to toxicity and makes the search for a more effective therapy with a shorter duration of chemotherapy necessary (36).
Antimalarial drugs have been shown to be effective against a broad range of parasitic diseases, such as trypanosomiasis, leishmaniasis, amoebiasis, and/or fungal infections (21, 37-40).
A few studies have been done on artemisinine and its derivatives against Echinococcus granulosus. Spicher et al. established the striking results of dihydroartemisinine and artesunate (10 to 40 µM) against Echinococcus granulosus and Echinococcus multilocularis protoscoleces in vitro and in vivo, although artemisinine and artemether were ineffective on protoscolics in their study (41). Xiao-juan showed that on the 9th day of incubation, an artemether high-dose group (100 μg/mL) killed 100% of Echinococcus multilocularis protoscoleces while the artemether low-dose (50 μg/mL) and albendazole groups had 93.28% and 99.03% mortality, respectively (42). As mentioned above, there is a discrepancy with regard to the effectiveness of are mether and artemisinine against hydatid disease because some authors have reported no metacestocidal effect (41). We showed that a high dose (200 μg/mL) of artemether killed all protoscoleces on day 4 of exposure; 100 μg/mL and 50 μg/mL killed the protoscoleces on the 6th and 7th days, respectively. The control groups exhibit no considerable alteration throughout the entire incubation period.
The artemisinine results were completely different and also exciting. Surprisingly, artemisinine (400 μg/mL) had no effect on protoscoleces up to the 8th day (95% viability, not shown in the figure). On the 9th day with 200 μg/mL of artemisinine, all protoscoleces were killed while they were going to dead protoscoleces after exposure to artemether, albendazole and artemisinine. This suggests that artemisinine had no effect on invaginated protoscoleces, but as they grow, this drug can affect them. It seems that there is a relation between the protoscoleces evaginated structure and artemisinine. According to the results of this work, artemisinine can be considered an effective drug for tissue and metacestode forms in human and animal models. Promising in vitro results were achieved with artemether and artemisinine and their combinations.
Because metacestodes are surrounded by a highly glycosilated acellular laminated layer, the drugs and their metabolites maynot be accumulated in the paracisitic tissues in sufficient quantities. Artemisinine and its derivatives have proved to be fairly safe (31). We believe that increased doses of safe drugs such as artemisinine can be used in vivo. The restructuring of the drugs to yield the best formulation can be attempted. Since for no ideal scolicidal agents have been presented (43). In vivo studies have considered the efficacy of albendazole-based combination therapy against echinococcosis. Rafiei et al. showed that albendazole and praziquantel combination therapy had greater antiechinococcal efficacy than albendazole alone (36).
It seems that artemether and artemisinine and their derivatives can be promising drugs as new scolicidal agents, butfurther investigationsare warranted (44). In vivo experiments of artemether and artemisinine also need to be conducted using an animal model.
Acknowledgements
References
-
1.
Eckert J, Deplazes P. Biological, epidemiological, and clinical aspects of echinococcosis, a zoonosis of increasing concern. Clin Microbiol Rev. 2004;17(1):107-35. [PubMed ID: 14726458].
-
2.
Matossian RM, Rickard MD, Smyth JD. Hydatidosis: a global problem of increasing importance. Bull World Health Organ. 1977;55(4):499-507. [PubMed ID: 74294].
-
3.
Arif SH, Shams Ul B, Wani NA, Zargar SA, Wani MA, Tabassum R, et al. Albendazole as an adjuvant to the standard surgical management of hydatid cyst liver. Int J Surg. 2008;6(6):448-51. [PubMed ID: 18819855]. https://doi.org/10.1016/j.ijsu.2008.08.003.
-
4.
Menezes da Silva A. Hydatid cyst of the liver-criteria for the selection of appropriate treatment. Acta Trop. 2003;85(2):237-42. [PubMed ID: 12606102].
-
5.
Ammann R, Eckert J. Clinical diagnosis and treatment of echinococcosis in humans. In: Thompson RCA, Limbery AJ, editors. Echinococcus and hydatid disease. Wallingford, UK: CAB International; 1995. 441–63 p.
-
6.
Bekhti A, Schaaps JP, Capron M, Dessaint JP, Santoro F, Capron A. Treatment of hepatic hydatid disease with mebedazole: preliminary results in four cases. Br Med J. 1977;2(6094):1047-51. [PubMed ID: 922416].
-
7.
Schantz PM, Van den Bossche H, Eckert J. Chemotherapy for larval echinococcosis in animals and humans: report of a workshop. Z Parasitenkd. 1982;67(1):5-26. [PubMed ID: 7041454].
-
8.
Morris DL, Dykes PW, Dickson B, Marriner SE, Bogan JA, Burrows FG. Albendazole in hydatid disease. Br Med J. 1983;286(6359):103-4. [PubMed ID: 6401475]. https://doi.org/10.1136/bmj.286.6359.103-a.
-
9.
Cobo F, Yarnoz C, Sesma B, Fraile P, Aizcorbe M, Trujillo R, et al. Albendazole plus praziquantel versus albendazole alone as a pre-operative treatment in intra-abdominal hydatisosis caused by Echinococcus granulosus. Trop Med Int Health. 1998;3(6):462-6. [PubMed ID: 9657508].
-
10.
Teggi A, Lastilla MG, De Rosa F. Therapy of human hydatid disease with mebendazole and albendazole. Antimicrob Agents Chemother. 1993;37(8):1679-84. [PubMed ID: 8215283].
-
11.
Pawlowski Z, Eckert J, Vuitton D, Ammann R, Kern P, Craig P. Echinococcosis in humans: clinical aspects, diagnosis and treatment. In: Eckert J, Gemmell MA, Meslin FX, Pawlowski ZS, editors. WHO/OIE manual on echinococcosis in humans and animals: a public health problem of global concern. Paris, France: World Organisation for Animal Health; 2001.
-
12.
Abdin MZ, Israr M, Rehman RU, Jain SK. Artemisinin, a novel antimalarial drug: biochemical and molecular approaches for enhanced production. Planta Med. 2003;69(4):289-99. [PubMed ID: 12709893]. https://doi.org/10.1055/s-2003-38871.
-
13.
Shetty P. Global fund switches to artemisinin. Lancet Infect Dis. 2004;4(8):477. [PubMed ID: 15298018].
-
14.
Krishna S, Uhlemann AC, Haynes RK. Artemisinins: mechanisms of action and potential for resistance. Drug Resist Updat. 2004;7(4-5):233-44. [PubMed ID: 15533761]. https://doi.org/10.1016/j.drup.2004.07.001.
-
15.
Efferth T. Willmar Schwabe Award 2006: antiplasmodial and antitumor activity of artemisinin--from bench to bedside. Planta Med. 2007;73(4):299-309. [PubMed ID: 17354163]. https://doi.org/10.1055/s-2007-967138.
-
16.
Utzinger J, Xiao S, Keiser J, Chen M, Zheng J, Tanner M. Current progress in the development and use of artemether for chemoprophylaxis of major human schistosome parasites. Curr Med Chem. 2001;8(15):1841-60. [PubMed ID: 11772354].
-
17.
Sen R, Bandyopadhyay S, Dutta A, Mandal G, Ganguly S, Saha P, et al. Artemisinin triggers induction of cell-cycle arrest and apoptosis in Leishmania donovani promastigotes. J Med Microbiol. 2007;56(Pt 9):1213-8. [PubMed ID: 17761485]. https://doi.org/10.1099/jmm.0.47364-0.
-
18.
Esavand Heydari F, Ghaffarifar F, Soflaei S, Dalimi A. Comparison Between in Vitro Effects of Aqueous Extract of Artemisia seiberi and Artemisinin on Leishmania major. Jundishapur J Nat Pharm Prod. 2013;8(2):70-5. [PubMed ID: 24624191].
-
19.
Mishina YV, Krishna S, Haynes RK, Meade JC. Artemisinins inhibit Trypanosoma cruzi and Trypanosoma brucei rhodesiense in vitro growth. Antimicrob Agents Chemother. 2007;51(5):1852-4. [PubMed ID: 17339374]. https://doi.org/10.1128/AAC.01544-06.
-
20.
Haynes RK. From artemisinin to new artemisinin antimalarials: biosynthesis, extraction, old and new derivatives, stereochemistry and medicinal chemistry requirements. Curr Top Med Chem. 2006;6(5):509-37. [PubMed ID: 16719805].
-
21.
Woodrow CJ, Haynes RK, Krishna S. Artemisinins. Postgrad Med J. 2005;81(952):71-8. [PubMed ID: 15701735]. https://doi.org/10.1136/pgmj.2004.028399.
-
22.
Keiser J, Utzinger J. Artemisinins and synthetic trioxolanes in the treatment of helminth infections. Curr Opin Infect Dis. 2007;20(6):605-12. [PubMed ID: 17975411]. https://doi.org/10.1097/QCO.0b013e3282f19ec4.
-
23.
Shalaby HA, Abdel-Shafy S, Abdel-Rahman KA, Derbala AA. Comparative in vitro effect of artemether and albendazole on adult Toxocara canis. Parasitol Res. 2009;105(4):967-76. [PubMed ID: 19468753]. https://doi.org/10.1007/s00436-009-1479-9.
-
24.
Morris DL, Chinnery JB, Georgiou G, Stamatakis G, Golematis B. Penetration of albendazole sulphoxide into hydatid cysts. Gut. 1987;28(1):75-80. [PubMed ID: 3817589].
-
25.
Scholar EM, Pratt WB. The antimicrobial drugs. 2nd ed. England: Oxford University Press; 2000.
-
26.
Lacey E. Mode of action of benzimidazoles. Parasitol Today. 1990;6(4):112-5. [PubMed ID: 15463312]. https://doi.org/10.1016/0169-4758(90)90227-U.
-
27.
Campbell WC, Blair LS. Treatment of the cystic stage of Taenia crassiceps and Echinococcus multilocularis in laboratory animals. J Parasitol. 1974;60(6):1053-4. [PubMed ID: 4436750].
-
28.
Eckert J, Barandun G, Pohlenz J. [Chemotherapy of echinococcosis in laboratory animals]. Schweiz Med Wochenschr. 1978;108(29):1104-12. [PubMed ID: 675204].
-
29.
Ceballos L, Elissondo C, Moreno L, Dopchiz M, Sanchez Bruni S, Denegri G, et al. Albendazole treatment in cystic echinococcosis: pharmacokinetics and clinical efficacy of two different aqueous formulations. Parasitol Res. 2008;103(2):355-62. [PubMed ID: 18465143]. https://doi.org/10.1007/s00436-008-0980-x.
-
30.
Kannan R, Kumar K, Sahal D, Kukreti S, Chauhan VS. Reaction of artemisinin with haemoglobin: implications for antimalarial activity. Biochem J. 2005;385(Pt 2):409-18. [PubMed ID: 15361062]. https://doi.org/10.1042/BJ20041170.
-
31.
Gera R, Khalil A. Artemisinine and its derivatives. Indian Pediatr. 1997;34(9):813-6. [PubMed ID: 9492419].
-
32.
Walker M, Rossignol JF, Torgerson P, Hemphill A. In vitro effects of nitazoxanide on Echinococcus granulosus protoscoleces and metacestodes. J Antimicrob Chemother. 2004;54(3):609-16. [PubMed ID: 15282238]. https://doi.org/10.1093/jac/dkh386.
-
33.
Ghaffarifar F, Esavand Heydari F, Dalimi A, Hassan ZM, Delavari M, Mikaeiloo H. Evaluation of Apoptotic and Antileishmanial Activities of Artemisinin on Promastigotes and BALB/C Mice Infected with Leishmania major. Iran J Parasitol. 2015;10(2):258-67. [PubMed ID: 26246824].
-
34.
Naguleswaran A, Spicher M, Vonlaufen N, Ortega-Mora LM, Torgerson P, Gottstein B, et al. In vitro metacestodicidal activities of genistein and other isoflavones against Echinococcus multilocularis and Echinococcus granulosus. Antimicrob Agents Chemother. 2006;50(11):3770-8. [PubMed ID: 16954323]. https://doi.org/10.1128/AAC.00578-06.
-
35.
Elissondo M, Ceballos L, Dopchiz M, Andresiuk V, Alvarez L, Bruni SS, et al. In vitro and in vivo effects of flubendazole on Echinococcus granulosus metacestodes. Parasitol Res. 2007;100(5):1003-9. [PubMed ID: 17171566]. https://doi.org/10.1007/s00436-006-0381-y.
-
36.
Rafiei A, Pipelzadeh MH, Jahanshahi A, Salim MRE. Comparing the effectiveness of albendazole and combination of albendazole and praziquantel in experimental hydatidosis. Arch Clin Infect Dis. 2009;4(1):9-12.
-
37.
Jefford CW. Why artemisinin and certain synthetic peroxides are potent antimalarials. Implications for the mode of action. Curr Med Chem. 2001;8(15):1803-26. [PubMed ID: 11772352].
-
38.
Kuster T, Stadelmann B, Hermann C, Scholl S, Keiser J, Hemphill A. In vitro and in vivo efficacies of mefloquine-based treatment against alveolar echinococcosis. Antimicrob Agents Chemother. 2011;55(2):713-21. [PubMed ID: 21135182]. https://doi.org/10.1128/AAC.01392-10.
-
39.
Soeiro Mde N, Dantas AP, Daliry A, Silva CF, Batista DG, de Souza EM, et al. Experimental chemotherapy for Chagas disease: 15 years of research contributions from in vivo and in vitro studies. Mem Inst Oswaldo Cruz. 2009;104 Suppl 1:301-10. [PubMed ID: 19753489].
-
40.
Keiser J, N'Guessan NA, Adoubryn KD, Silue KD, Vounatsou P, Hatz C, et al. Efficacy and safety of mefloquine, artesunate, mefloquine-artesunate, and praziquantel against Schistosoma haematobium: randomized, exploratory open-label trial. Clin Infect Dis. 2010;50(9):1205-13. [PubMed ID: 20350194]. https://doi.org/10.1086/651682.
-
41.
Spicher M, Roethlisberger C, Lany C, Stadelmann B, Keiser J, Ortega-Mora LM, et al. In vitro and in vivo treatments of echinococcus protoscoleces and metacestodes with artemisinin and artemisinin derivatives. Antimicrob Agents Chemother. 2008;52(9):3447-50. [PubMed ID: 18625777]. https://doi.org/10.1128/AAC.00553-08.
-
42.
Xiao-juan L, Hua Y, Gen-Shu B. In vitro observation of artemether's efficacy against Echinococcus multilocularis protoscoleces [J]. J Pathog Biol. 2011;2:18.
-
43.
Adas G, Arikan S, Kemik O, Oner A, Sahip N, Karatepe O. Use of albendazole sulfoxide, albendazole sulfone, and combined solutions as scolicidal agents on hydatid cysts (in vitro study). World J Gastroenterol. 2009;15(1):112-6. [PubMed ID: 19115476].
-
44.
Mohammadnejad F, Ghaffarifar F, Dalimi A, Hassan ZM. The development of new treatment options for echinococcosis. Rev Med Microbiol. 2015;26(4):154-62. https://doi.org/10.1097/mrm.0000000000000058.