1. Background
Idiopathic pulmonary fibrosis remains a fatal disease with a highly mortality and morbidity rate, as no safe and efficacious agent for its treatment has been discovered yet (1). Fibrosis is defined as the accumulation of components of the extracellular matrix, such as fibronectins, collagen proteins, and hyaluronic acid, at the site of the injured tissue; fibrosis results in decreased function and failure of the organ and finally death (2). Tissue or organ fibrosis is generally considered as an uncontrolled wound healing process (3). Acute distress syndrome caused by acute lung injures and traumas results in chronic interstitial pulmonary fibrosis, which is characterized by progression of fibrosis and collagen deposition (4).
Although the precise mechanism involved in lung fibrosis is poorly understood, it is believed to be the end stage of the repairing process that occurs after physical, chemical or biochemical lung injures and is characterized by progressive scarring of lung tissue. A variety of inflammatory cytokines and chemokines play a major role in amplifying, mediating, and perpetuating the lung injury and fibrotic process (5). Among them, transforming growth factor beta (TGF-β) is considered as the major profibrotic cytokine, which promotes proliferation, the conversion of fibroblasts to myofibroblasts, and finally, the deposition of extracellular matrix proteins, orchestrating fibroblast function as a chemotactic factor (3). A large body of studies has documented the modulating role of angiotensin-converting enzyme (ACE) and its related peptide, angiotensin II (ANG II), in the pathogenesis of lung fibrosis (6). The close association of ANG II with fibrogenesis in the circulatory system and tissue injuries was shown by a recent study (7). ANG II is produced by activation of the local renin-angiotensin system, where it is believed to play a decisive role in remodeling and repairing via the action of TGF-β, at least in part. Although the fibro-proliferative actions of ANG II in the lung have not been characterized, in vitro studies have demonstrated that ACE inhibitors can abrogate experimental pulmonary damages via a variety of mechanisms. Moreover, it is hypothesized that after lung injuries, ANG II can stimulate fibroblasts to produce pro-collagen and promote collagen deposition in the lungs of rats (8). Although the main function of the renin angiotensin system and its effectors is blood pressure regulation, it plays a key role in promoting inflammatory cascades. Inflammation is a crucial mechanism in the progression and development of fibrotic processes, and the rennin-angiotensin system has profibrotic and proinflammatory effects at the cellular and molecular levels (9). During the experimental lung injury, ACE inhibitors were found to attenuate the activation of tumor necrosis factor (TNF) and endothelial cells and the deposition of collagen proteins, possibly by decreasing the apoptosis rate of epithelial cells (8). Therefore, blocking this system and its related effectors or receptors could prove to be beneficial for the prevention and treatment of inflammatory events. There is a growing list of drugs and chemical agents, including antibiotic, immunosuppressive, antiarrhythmic, and chemotherapeutic agents and mineral and industrial dust, which have been implicated in lung injury and the resulting pulmonary fibrosis (10).
Bleomycin is an antibiotic chemotherapeutic agent that was investigated in 1976 by the Umezawa group. It has a decisive effect in the treatment of germ cell cancers, squamous-cell carcinomas, cancerous pleural effusion, and lymphomas. However, since fibrosis is one of the critical side effects of bleomycin, it is used extensively for inducing pulmonary fibrosis in experimental rodent animals (4, 11-16). The exact mechanism involved in the progression of pulmonary injury after bleomycin administration is not well understood (11). Bleomycin-induced lung fibrosis appears to be the consequence of primary inflammatory bruises characterized by an aggregation of alveolar neutrophils and macrophages in the lower pulmonary tract. Several studies have indicated that ROS, superoxide anions (17) in particular, play an indisputable role in these events (18).
Medicinal plant derivatives are traditionally used to treat a variety of conditions, including pain, inflammation, fever, asthma, hypertension, liver disorders, and malignancies. For example, Hibiscus sabdariffa L. (Roselle; Malvaceae), which is known to contain several antioxidants such as protocatechuic acid (PCA) and anthocyanins, has potential as an ethnomedical remedy for diabetes, jaundiced liver, hypertension, obesity and urinary dysfunctions. These effects have been confirmed in humans and experimental animals (19). Recent studies have demonstrated that H. sabdariffa L. extract (HSE) exhibits antioxidant, antidiabetic, antibacterial, antihypertensive, antiproliferative, anticholesterol, nephroprotective, cardioprotective, hepatoprotective and renal-diuretic activities (20, 21). These properties may be attributable to its inhibitory effects on α-amylase and α-glucosidase, and its potent antioxidant, direct vaso-relaxant, Ca++ channel modulatory and, especially, ACE inhibitory effects. In addition, anthocyanins (mainly cyanidin- and also delphinidi-3-sambobiosides), phenolic acids (especially PCA) and organic acids (such as hydroxycitric acid and hibiscus acid) may also contribute to the aforementioned effects (21). In several countries, infusions and decoctions of roselle calyces and even leaves are used for the treatment of hypertension or hypercholesterolemia without any recorded adverse or toxic effects (LD50, 2 - 5 g kg-1 day-1) (22).
2. Objectives
The purpose of this study was to investigate the inhibitory effect of HSE on ACE and to compare this effect to that of enalapril in a bleomycin-induced rat model of pulmonary fibrosis.
3. Methods
3.1. Chemicals
1, 1, 3, 3-Tetramethoxypropane (TEP), thiobarbituric acid (TBA), trichloro acetic acid (TCA), 3,4-dihydrobenzoic acid (PCA), L-hydroxyproline, bovine serum albumin (BSA) and Bradford reagent were purchased from Sigma-Aldrich chemical company (St. Louis, MO, USA). Bleomycin sulfate (15 U/vial) was gifted by Nippon Kayaku Ltd., Tokyo, Japan, and enalapril was also gifted by Irandaroo company. The ELISA kits for TGF-β1, platelet-derived growth factor (PDGF), TNF-α and ANG II were purchased from eBioscience company, USA. All the chemicals and reagents used were of analytical grade.
3.2. Extract Preparation
Fresh and well dried calyces of H. sabdariffa L. were identified at the Department of Pharmacognosy, School of Pharmacy, Ahvaz, Iran. The dried plant was soaked in a 70% aqueous-ethanol solution in a large container for 3 days with occasional shaking (23-25). The solvent was filtered through a Whatman paper and was then extracted under vacuum until dry in a rotary evaporator at 40°C (26, 27).
3.3. Animals
Adult female Wistar albino rats (weighing 180 - 235 g) were obtained from the animal house of Ahvaz Jundishapur University of Medical Science, Iran. The mice were kept in polypropylene cages and provided with standard rat chow and drinking water ad libitum. The animals were maintained under controlled conditions at a temperature of 20 ± 2°C with a 12-hour light:12-hour dark cycle. The animals were acclimated to the environment for a minimum of one week prior to inclusion in the experiment. The investigation was performed according to the guidelines of the animal ethics committee for the use of experimental animals (Ethics approval number: 1394.2.14).
3.4. Experimental Design
The animals were randomly divided into seven groups (n = 8). The negative and positive control groups were intratracheally administered a single dose of saline (1 mL/kg) and bleomycin (7.5 UI kg-1 mL-1) (28) respectively. The treatment groups were orally administered enalapril (20 mg/kg), PCA (100 mg/kg) and HSE (100, 200, and 400 mg/kg) (29) daily one week before and three weeks after bleomycin administration.
3.5. Sample Collection
On the 22nd day, 24 hours after the last administration, the animals were anaesthetized with ketamine/xylazin. Bronchoalveolar lavage fluid (BALF) samples were obtained by instilling 1 mL chilled sterile normal saline three times (total yield, nearly 2.5 mL). Each lung BALF sample was centrifuged at 300 g for 10 minutes at 4°C immediately, and the supernatants were refrigerated at -80°C for analysis of pro-inflammatory molecules. The lungs were resected, washed and weighed quickly. For histological studies, the whole left lobe of the lungs was fixed in 10% phosphate buffered formalin. The lung lobes were then separated, and the upper and lower lobes of the right lung were used for biochemical analyses. The lung tissues were homogenized (1/10 w/v) in ice-cold Tris-HCl buffer (0.1 M, pH 7.4), and the protein content of the homogenates was determined using the Bradford method (30), with crystalline BSA as the standard.
3.6. Lung Index
The body weight of the rats was measured every 7 days. After the rats were sacrificed, the lung index was expressed as the ratio of the wet lung weight (mg) to body weight (g) (31).
3.7. Lipid Peroxidation Assay
Lipid peroxidation was examined by measuring the amount of malondialdehyde (MDA) via the TBA color reaction by the method described by Buege and Aust (32). Briefly, 0.5 ml of lung homogenate was mixed with 2.5 ml of TCA (10%, w/v), the samples were centrifuged at 3000 rpm for 10 min, and 2 ml of each sample supernatant was transferred to a test tube containing 1 ml of TBA solution (0.67%, w/v). The mixture was kept in boiling water for 10 min to induce the formation of a pink colored solution. The mixture was then cooled immediately, and absorbance was measured at 532 nm with a spectrophotometer. TEP was used as the standard, and the MDA content was expressed as nmol/mg protein.
3.8. Hydroxyproline Assay
The total collagen content of the lung was measured using a colorimetric assay to determine the lung hydroxyproline (HP) content (33, 34). In brief, the minced left lung lobes were homogenized in 6 mol/l HCl and hydrolyzed for 5 hours at 130°C. The pH was adjusted to 6.5 - 7.0 with NaOH, and the sample volume was adjusted to 30 mL with distilled water. The sample solution (1.0 mL) was mixed with 1.0 mL of chloramine T solution (0.05 mol/L), and then the mixture was incubated at room temperature for 20 min. When 1.0 ml of 20% dimethyl benzaldehyde solution was added, the mixture was incubated at 60°C for 20 minutes. The absorbance of each sample at 550 nm was measured. The results were calculated as the weight of HP in micrograms per gram of wet lung tissue.
3.9. ELISA
The TGF-β1, PDGF, TNF-α and ANG II peptide levels in the BALF samples were measured using specific ELISA kits according to the manufacturer’s instructions.
3.10. Histological Examination
The left lung was rewashed and fixed in a 10% formaldehyde solution for at least 48 hours. Consequently, it was embedded in paraffin, and 5-μm-thick sections were cut with a microtome and placed on slides for Masson’s trichrome staining.
3.11. Statistical Analysis
Results were expressed as the mean ± SEM values, and all the statistical comparisons were conducted using one-way ANOVA followed by Tukey’s post-hoc analysis. Data analysis was performed using Prism 5.0 (San Diego, CA, USA). P values less than 0.05 were considered to indicate statistical significance.
4. Results
4.1. Lung Index
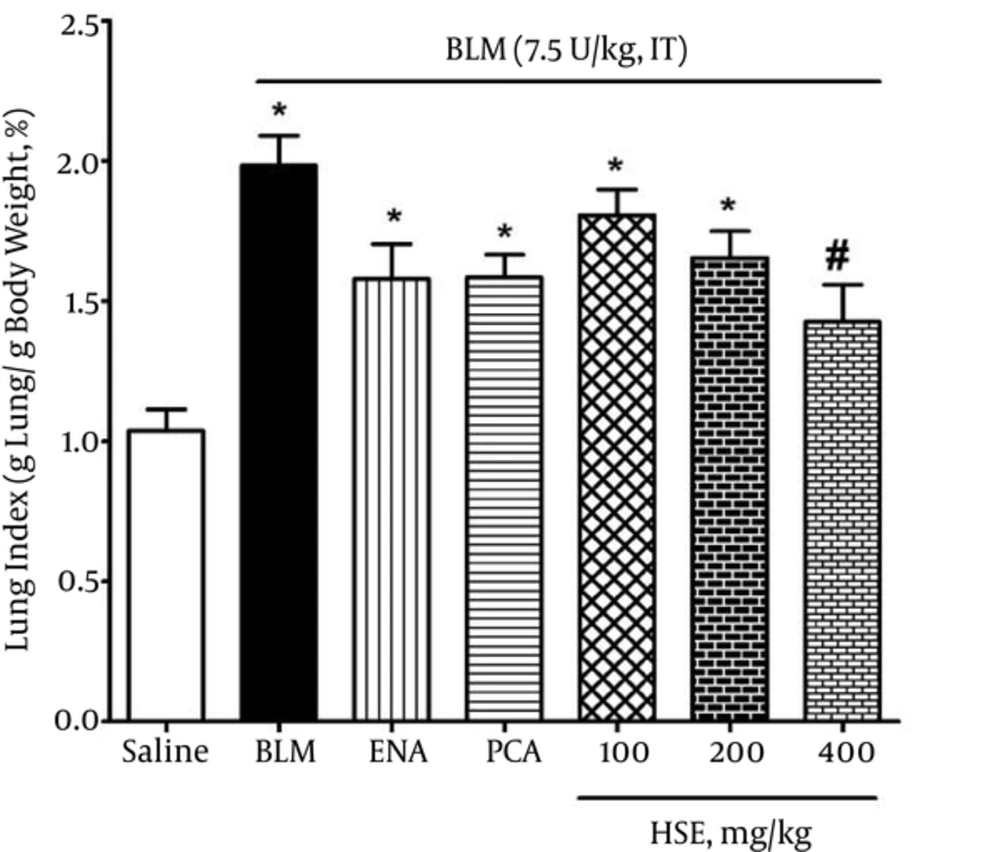
The body weight in the bleomycin group was significantly lesser than that in the saline group: this was indicative of lung fibrosis in the bleomycin group. Moreover, the lung index in the bleomycin group was significantly higher than that in the saline group on day 28 (P < 0.05). However, the rats that were administered 400 mg/kg HSE had a significantly lower lung index than the rats in the bleomycin group (P < 0.05) (Figure 1).
4.2. MDA Levels
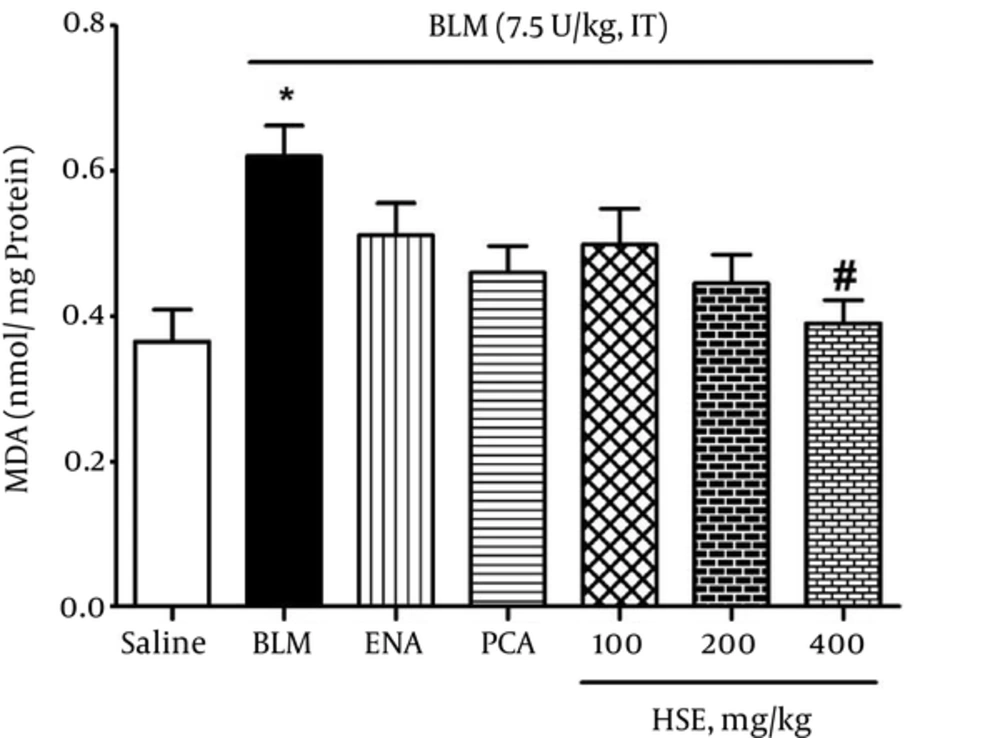
The TBARS assay showed that the MDA levels had almost doubled at 3 weeks in the lung tissue of rats treated with bleomycin (0.62 nmol/mg protein); moreover, the MDA levels were significantly higher than those in the saline group (0.365 nmol/mg protein) (P < 0.05). However, in the treatment groups, HSE administered at a dose of 400 mg/kg resulted in a marked decrease in the MDA levels (0.39 nmol/mg protein) so that the MDA level reached that of the control group (P < 0.05) (Figure 2).
4.3. HP Content
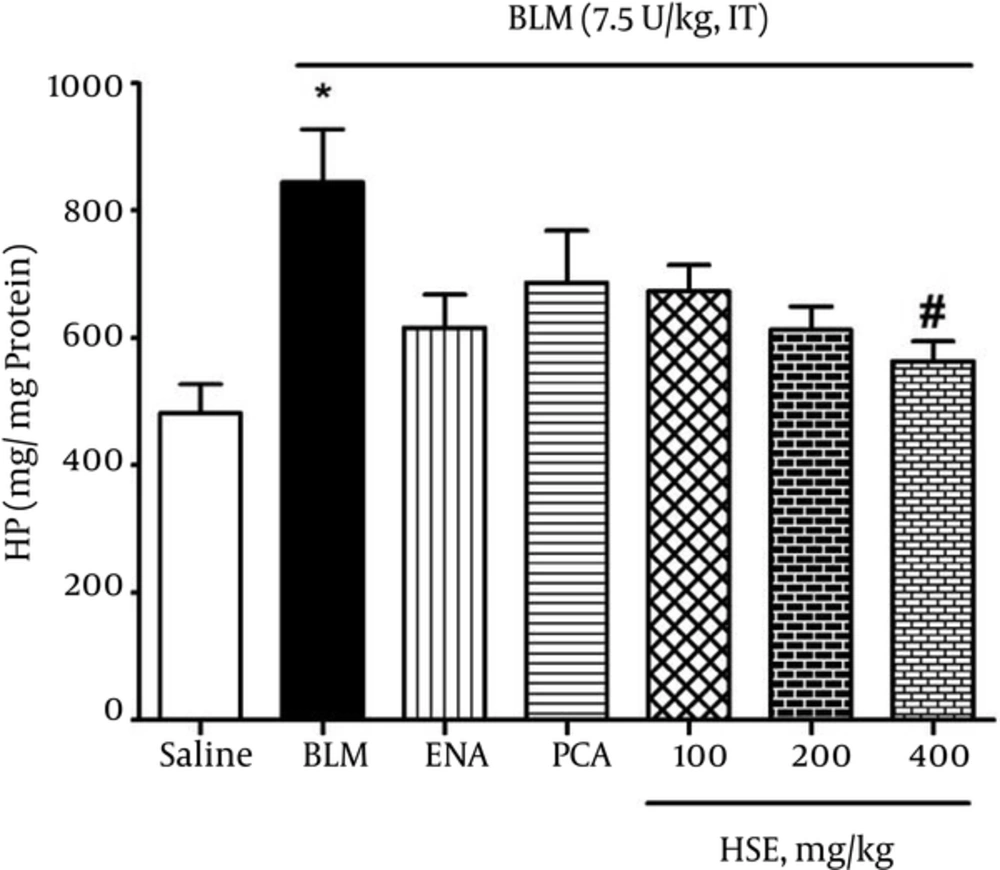
The HP content in lung tissues was investigated as an index of collagen deposition. As shown in Figure 3, the data showed that the lung HP content of bleomycin-treated rats (844 μg/g lung) was significantly greater than that of the saline group (482 μg/g lung). Further, administration of HSE (400 mg/kg) significantly reduced the HP content in lung tissue (P < 0.05).
4.4. Cytokine Levels
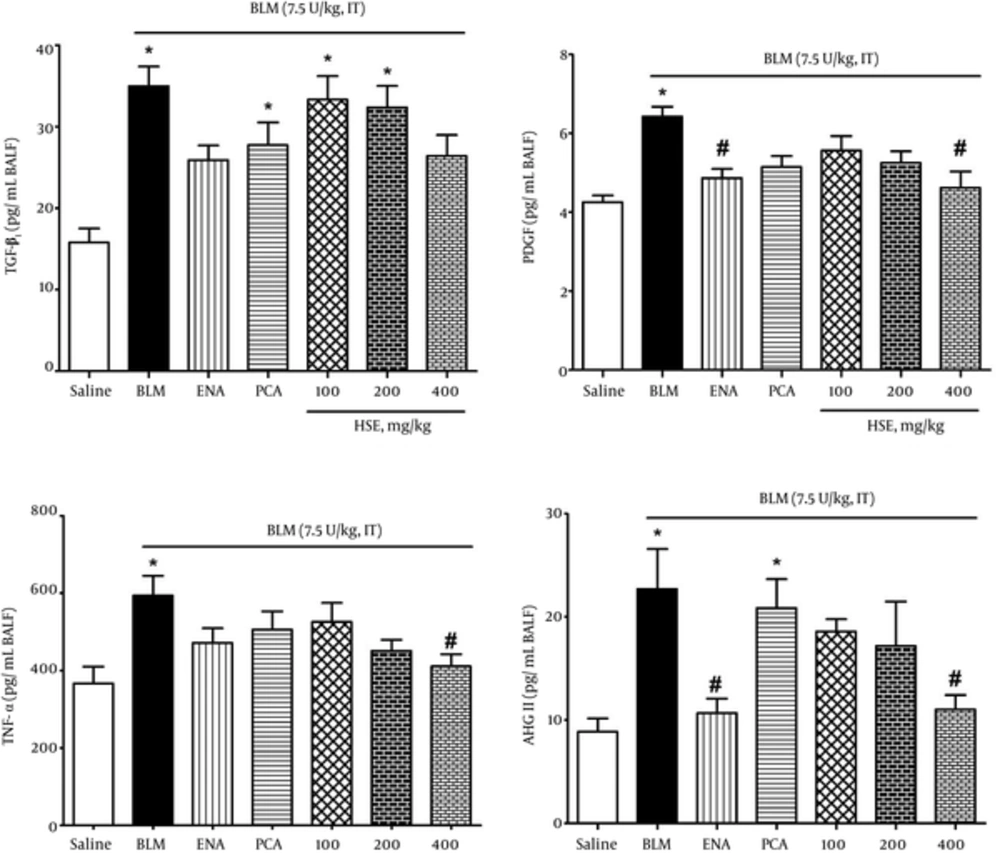
To evaluate the effect of enalapril, PCA and HSE on bleomycin-induced lung injury, we measured the levels of the important pro-inflammatory and profibrotic cytokines PDGF, TGF-β1, TNF-α and ANG II peptide in BALF samples. Administration of bleomycin caused a significant elevation in the levels of the above factors as compared with the saline group. Treatment of rats with BVFE reduced the bleomycin-induced production of PDGF, TGF-β1, TNF-α and ANG II peptides (Figure 4).
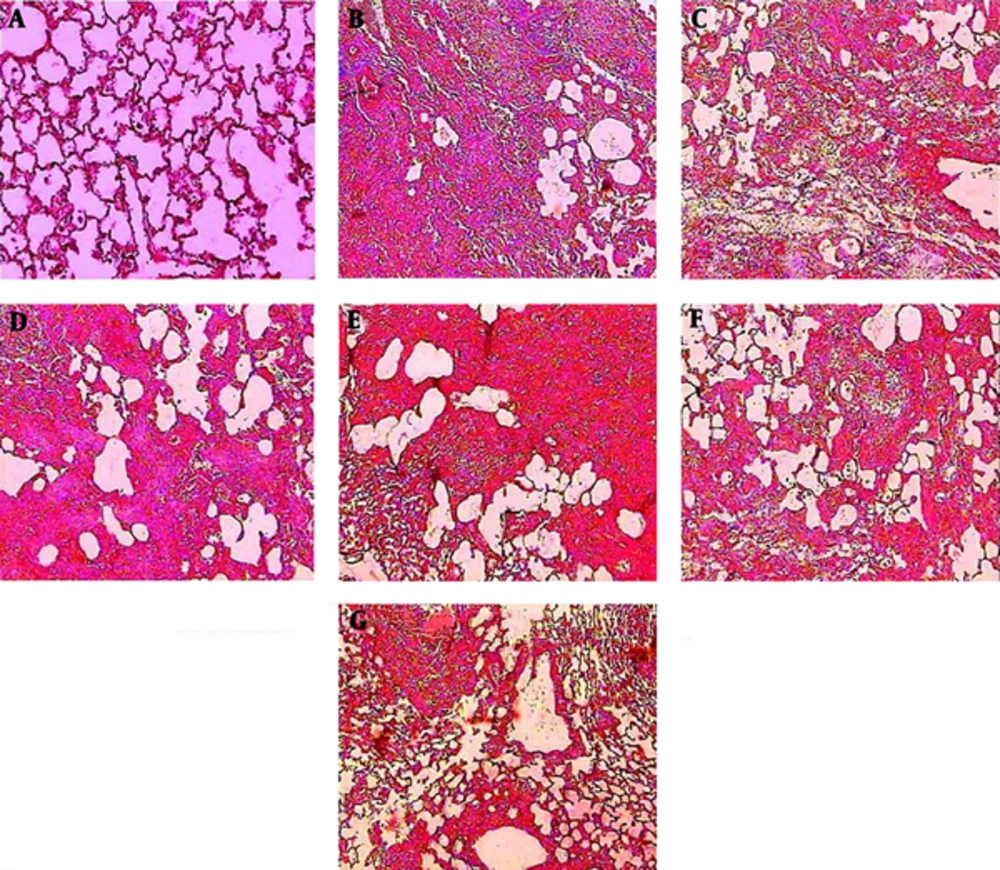
4.5. Histological Observations
Histopathological evaluations after Masson trichome staining of sections prepared from the dissected left lobe of rat lungs showed significant changes between the bleomycin and saline group. Thickening of the wall and interstitium between alveolar sacks due to deposition of ECM and migration and proliferation of macrophages, neutrophils and fibroblasts were obvious. Oral treatment with PCA, enalapril and HSE caused relative modifications in collagen deposition in the alveolar cell walls and interstitium. Marked reduction in wall thickness and alveolar interstitium was observed in the groups that were administered HSE at the dose of 400 mg/kg. Moreover, moderate amelioration in inflammatory events and collagenous sedimentation was observed in the groups treated with 200 mg/kg HSE and enalapril (Figure 5).
Lung tissues were stained with Masson trichrome 21 days after the administration of BLM. The appearance of the rat lungs in different groups: (A) saline, (B) BLM, (C) BLM treated with ENA (20 mg/kg), (D) BLM treated with PCA (100 mg/kg), (E, F, G) BLM treated with HSE (100, 200 and 400 mg/kg). The alveolar structure in the saline group (A) was normal. An image of a fibrotic lung induced by BLM on day 21 showing extensive inflammatory cell infiltration, alveolar thickening and diffuse fibrotic changes with patchy areas of interstitial collagen deposition (B). An image of the lungs of the groups treated with ENA (C) and HSE (400 mg/kg) (G) showing fewer infiltrates of inflammatory cells. In the group treated with PCA (D), the observations were the same as those in the HSE group (100 and 200 mg/kg) (E & F) but better than those in the BLM group.
5. Discussion
In the lung, inflammatory cascades commence following alveolar epithelial damage, and the severity of the fibrogenic response is directly related to the severity of alveolar epithelial cell injury. Indeed, a key event in the pathogenesis of lung fibrosis is apoptosis of alveolar epithelial cells (14). This is followed by the increased expression of profibrotic cytokines and increase in extracellular matrix deposition, which result in the development of fibrotic lesions (fibroblast “foci”). Consequently, a large variety of growth factors, cytokines and matrix metalloproteinases are released by the injured epithelial cells (35). This is accompanied by the activation, proliferation and migration of mesenchymal cells, which is believed to be the end-stage process that is responsible for organ failure and dysfunction (11). AT1 receptor antagonists and ACE inhibitors have proven to be important for the treatment of disorders in which tissue remodeling and fibrosis occur, such as cardiovascular and renal disease. Further, it has been shown that ANG II is an influential activator and stimulator of in vitro collagen production by lung fibroblasts: this function is partly mediated by autocrine modulation of TGF-β via the AT1 receptors (36). In the present study, through induction of the fibrosis model by bleomycin, we assessed the protective and anti-fibrotic effects of a natural ACE inhibitor on lung fibrosis, and compared it with the effects of the standard synthetic ACE inhibitor.
The data of the present study showed that the natural ACE inhibitor HSE had an ameliorative effect on the lung index (Figure 1), as reflected by the reduction in body weight and increase in lung weight. Loss of appetite, difficulty in breathing and the production of inflammatory factors may be considered as the main reasons for weight loss in the bleomycin group. Because of infiltration and proliferation of the lung epithelial cells and massive collagen deposition, lung weight was considerably higher in the bleomycin-treated rats than in the control rats. In this study, treatment with HSE could significantly improve the lung index, as reported by a recent previous study about PCA (37): this effect may be brought about by a reduction in the number of total cells, neutrophils, macrophages and protein concentration in the BALF or improvement in appetite.
In the present study, the lung MDA equivalent was significantly lower in the treatment groups than in the bleomycin group (Figure 2). A growing number of studies have suggested that the use of antioxidants can reduce the inflammatory response generated by ROS in animals (18, 38, 39). Further, a large number of both in vitro and in vivo studies have shown that extracts of the calyx of roselle flowers have potent antioxidant activity (40, 41). Phenolic compounds, however, play a protective role against oxidative damage to important biomolecules via free-radical scavenging and their potent antioxidant properties (41). In accordance with these studies, the present study showed that HSE (administered at a dose of 400 mg/kg) can significantly reduce oxidative stress events via several antioxidants such as anthocyanins and its bioactive ingredient PCA through its high antiradical activity (19, 39). The results showed that there is no significant difference between the group administered 400 mg/kg HSE and the control group with regard to the lung tissue MDA levels.
In our study, the data indicated that HSE treatment attenuated lung collagen accumulation, as depicted by the noticeable reduction in the lung HP content (Figure 3) and the decrease in the PDGF, TGF-β1 and ANG II levels in BALF samples in bleomycin-induced lung fibrosis (Figure 4). In agreement with these results, treatment with HSE, enalapril and PCA also resulted in the amelioration of histopathological scores in a dose-dependent manner: there was a significant difference in the Ashcroft scores between the HSE-treated (high dose, i.e., 400 mg/kg) and bleomycin groups (Figure 5). Previous studies have indicated that an increase in expression of the pro-collagen I gene and TGF-β1 where terminate to collagen deposition is observed from the 9th day after bleomycin treatment (13, 42). Although normal wound healing is brought about via complex interactions between pro-fibrotic and anti-fibrotic cytokines, chemokines and other cell mediator proteins, tissue fibrosis is considered as one of the mechanisms underlying uncontrolled and confused wound healing (3). Fibroblasts are activated and differentiate into myofibroblasts during both physiological and pathological wound repairing processes. In vitro and in vivo studies have demonstrated that TGF-β1 is a key growth factor in driving the differentiation of fibroblasts into myofibroblasts and finally fibrosis (2). In the present assessment, the ANG II, TGF-β1 and PDGF levels in BALF samples of the HSE- and also enalapril-treated group decreased significantly (Figure 4). Although HSE (400 mg/kg) was observed to markedly reduce the TNF-α level, other treatments could also effectively decrease it. This finding also indicates that PCA may have a better impact on the modification or suppression of gene expression of PDGF than TGF-β1. A recent study has suggested that PCA analogues can potentially confer anti-fibrotic effects through the inhibition of TGF-β1, CTGF, and type I and III collagen (43, 44). In keeping with the main aim of the study, which was to study the effects of these treatments on ACE inhibition, it was found that both HSE and enalapril but not PCA could potently and significantly reduce the BALF ANG II levels. This finding may indicate that despite the dominant antioxidant activity of PCA, it may not be able to inhibit ACE.
There is a large body of evidence which indicates that ANG II activates the ANG II type 1 receptor and is involved in multiple models of fibrosis (14). Couluris’ study performed in 2010 showed that ANG II receptors are involved in the synthesis of pro-collagen via promotion of lung fibroblast proliferation, which is brought about by induction of TGF-β expression (1). Meantime we would expect to see a synergistic effect in blocking of the ANG II generation by a capable compound which has the constituents with strong antioxidant and free radical scavenging capacities. As mentioned before, roselle calyces, in addition to the vast quantities of antioxidants, also contain biomaterials with ACE inhibitory effects, such as anthocyanins (mainly cyanidin and delphinidi-3-sambobiosides). The levels of inflammatory cytokines and pulmonary biochemical parameters, such as the hydroxyproline (collagen index) and malondialdehyde level, and also histopathological assessment indicate that HSE controls inflammatory processes in a dose-dependent manner.
5.1. Conclusion
It appears that the ACE inhibitory potency of HSE is similar to that of enalapril (as shown in Figure 4). Therefore, based on the results of previous studies and our experimental data, we can assume that the ACE inhibitory effect of HSE (administered at a dose of 400 mg/kg) is similar to that of a standard synthetic ACE inhibitor (enalapril). We also concluded that HSE, which has been shown to have anti-hypertensive effects in herbal medicine, may be useful in the treatment of inflammatory and fibrotic disorders.