Abstract
Background:
Marine organisms such as Echinoderms have secondary metabolites, which are antimicrobial naturally. Various extracts of echinoderms organs possess pharmacological activities, including anticancer, antimicrobial, antifungal, antiviral, and anti-inflammatory functions.Objectives:
The purpose of this research was to assay the Persian Gulf sea urchin secondary metabolites for antimicrobial effectiveness.Methods:
Sea urchins (Echinometra mathaei) were collected from the Boushehr tide coasts, Persian Gulf. Gonads, gut, tests, shell, and spines were carefully dissected from sea urchins, washed with tap water, and separated for the extraction procedure. All organs were extracted with 1:3 volumes (v/w) of methanol, chloroform, and n-hexane by maceration method for 72 hours at room temperature. The antimicrobial activity of the sea urchin tissue extracts was tested by well diffusion agar method.Results:
All extracts of the sea urchin E. mathaei at 50 mg/mL concentration exhibited in vitro antimicrobial activity against Gram-negative and Gram-positive bacteria, including Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, and Bacillus subtilis, and two fungi, including Candida albicans and Aspergillus niger. The antibacterial activity of the sea urchin extracts varied with the solvent used for the bacterial strains.Conclusions:
The results clearly showed the high antimicrobial activity of test and spines of the Persian Gulf sea urchin extracts against various Gram-positive and Gram-negative bacteria.Keywords
Sea Urchin Echinometra mathaei Persian Gulf Antimicrobial Activity Organic Extract
1. Background
The ocean covers around 70% of the earth’s surface. Humans, particularly depend on marine systems for a high number of their practices such as food resources, ways to travel around, business, and more recently as a source of important metabolites for the cosmetic and pharmaceutical industries. In recent decades, high bioactivity studies on compounds isolated from marine organisms have turned sea life into a new and prolific source of metabolites that can be very efficient to improve human health and life quality (1).
In recent years, many bioactive compounds have been extracted from different marine organisms, including gastropods, tunicates, sponges, soft corals, sea hares, sea cucumbers, sea urchins, and bryozoans. The number of natural products isolated from marine organisms is increasing rapidly, and now exceeds hundreds of new compounds being discovered every year (2).
Secondary metabolites are not essential to the life of the producing organism and are formed from primary metabolites. The secondary metabolites have various functions, with pharmacological activities, including anticancer, antimicrobial, antifungal, antiviral, and anti-inflammatory functions, and are potential sources as new therapeutic agents (3).
The majority of pharmacologically active secondary metabolites have been isolated from echinoderms (4). Echinoderms seem to have secondary metabolites, which are antimicrobial naturally. Echinoderms are invertebrates and include a number of species with significant roles in the marine ecosystem (5). Among the sea creatures, sea urchin is a large and diverse group of which many secondary metabolites are extracted. Different species of sea urchins are extensively distributed throughout oceans worldwide (6). The urchin populations are concentrated in shallow water and their densities can be as high as 350 urchins per m2 (7).
Like sea urchins, echinoderms belong to the class of Echinoidea and the phylum of Echinodermata, which are found on the sea floor worldwide (8). Sea urchins or urchins have a hard calcareous shell called “test”, which is covered with a thin epithelium and is usually armed with spines. Sea urchins have a smile anatomic structure. Intestine, gonads, nerve ring, as well as other organs, which are protected by a hard skeleton form the coelomic cavity.
The antimicrobial activity in several species of echinoderms collected from the Gulf of California, Mexico, Caribbean, and Coast of Norway has been reported (9-11). Also, a variety of antimicrobial factors, including steroidal glycosides (12). Hydroxylated sterols (13), lysozymes (14, 15), complement-like substances (16), and antimicrobial peptides (17) have also been isolated from echinoderms (18).
Antibacterial activity has previously been described in a wide range of echinoderm species (11, 19-21). Anbukkarasu et al. studied the antimicrobial properties of starfish Luidia maculate extract (22). Moreover, Haug et al. studied the antibacterial activity of different parts of the sea urchin Strongylocentrotus droebachiensis, star fish Asterias rubens, and the sea cucumber cucumaria frondosa against human pathogenic bacteria (9). In addition, Stabili et al. studied the antibacterial activity in the coelomocytes of the sea urchin Paracentrotus lividus (15). Further, Shankarlal et al. studied the antimicrobial and antioxidant activity of purple sea urchin shell Salmacis virgulata; they showed that the purple sea urchin S. virgulata has potential antimicrobial properties against the Vibrio cholera and Salmonella typhi and proteus species. It may be used in research on urinary tract infections (23). Moreover, hexane extract of T. Alexandri has been proven to have very good antibacterial activity against many bacteria (24).
In a recent study, the antibacterial compound was shown to be the lysozyme. The antimicrobial activity has been found in eggs of other marine invertebrates as well (9, 25) and both of these studies showed that at least some of the antibacterial compounds are not proteinaceous (18). As antibacterial activity has been demonstrated against both Gram-positive and Gram-negative bacteria, as well as selected fungal species, thus it may be reasonable to assume that multiple factors are responsible for the antimicrobial activity (18).
A variety of antimicrobial compounds, including steroidal glycosides (13, 26), polyhydroxylated sterols (13), naphthoquinone pigments (27), lysozymes (14, 15), complement-like substances (16), and antimicrobial peptides (28) have been isolated from echinoderms. More recently, antibacterial and hemolytic effects of aqueous and organic extracts from different tissues of sea urchin Echinometra mathaei against pathogenic streptococci has been reported (29). Wide variety of bioactive compounds showed the presence of various substances that have antimicrobial effectiveness. Therefore, marine echinoderms can be considered sustainable resources for the discovery of new antibiotic compounds.
2. Objectives
The present study focused on screening and comparing the antimicrobial activity of the body wall, gonads, tests, and gut extracts of the sea urchin E. mathaei collected from the Persian Gulf (Boushehr coastal) on Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, Bacillus subtilis, and Candida albicans microorganisms.
3. Methods
3.1. Sample Collection
The sea urchins were randomly collected from the Boushehr tidal coasts, the Persian Gulf in July 2016. All samples were transported to the laboratory for identification and characterization. All chemicals at analytical grades were obtained from Merck Company, Germany.
The scientific identification of each animal was determined before dissection. Gonads, gut, tests, shell, and spines were carefully dissected from 33 sea urchins, washed with tap water, and separated for the extraction. Components isolated from the sea urchins were pooled and preserved in methanol, chloroform, and hexane, separately. The samples were then stored for 72 hours in the dark at room temperature to avoid photolysis and thermo degradation of secondary metabolites prior to extraction.
3.2. Specimen and Sex Determination
Specimen identification was performed with the use of the tests and spines of the sea urchin. After taking digital photographs of test and spines through a stereomicroscope, the identification of the species was done with the diagnostic keys (30). The sex determination and scientific identification of sea urchins were carried out by Marine Science and Technology University of Khoramshahr. Echinometra mathaei was identified as scientific species.
3.3. Preparation of Extracts
The extraction was performed as described by Abubakar et al. with slight modifications. Separate portions of Gonads (89 g), gut (46 g), test, and spines (33 g) of E. mathaei were extracted with 1:3 volumes (v/w) of methanol, chloroform, and n-hexane by maceration method for 72 hours at room temperature. The resulting solution was filtered through a cotton sterile filter. The extract was concentrated using a rotary evaporator at 40°C. The crude extracts were obtained by freeze-drying and stored at -20°C.
3.4. Microorganisms and Culture Media
All microorganisms i.e. Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, Bacillus subtilis, Aspergillus niger and Candida albicans were obtained from the Microbiology Laboratory of Ahwaz Golestan Hospital. All isolated bacteria were grown on nutrient agar and maintained at 37°C for 24 hours.
3.5. Antimicrobial Assay
The antimicrobial activity of the sea urchin tissue extracts was tested by well agar diffusion method. The bacteria were cultured on nutrient agar for 24 hours (31). For antibacterial activity, 20 mL of sterile nutrient agar was poured in each petri plate, allowed to set at 37°C for 24 hours. Cultures were swabbed in nutrient agar plates by using a sterile cotton swab aseptically. All experiments were conducted in triplicate. The mean and standard deviation were compared by one way ANOVA.
For antifungal activity, 20 mL of Sabouraud agar were poured and allowed to set before inoculating uniformly with 0.1 mL of 48-hour broth culture of test fungi. The organic extracts with 5 concentrations were prepared by transferring 10, 20, 30, 40, and 50 mg of each extract to 1mL of dimethyl sulfoxide (DMSO) (Merck, Germany). Then each extract was transferred to a well of 7 mm diameter punched in swabbed plates and incubated at 37°C for 24 hours. Antibacterial activity was determined by measuring the diameter of the inhibitory zone (mm). Antibiotics such as vancomycin, tetracycline, penicillin, gentamycin, ceftriaxone, and amoxicillin were used as positive controls. DMSO, methanol, chloroform, and n-hexane were also tested as a negative control to ensure that they do not interfere with the tests. The organic extracts of E. mathaei showed considerable antibacterial activity was selected for determination of MIC (32).
3.6. Minimum Inhibitory Concentration (MIC)
The MIC was determined by using the microdilution method, using (12 × 8 wells) microtiter plates. A solution containing 50 mg/mL E. mathaei was prepared (32). Aliquots (50 µL) of the E. mathaei crude extract and 200 µL of Mueller Hinton broth were transferred to the well-labeled as A. Only 100 µL of Mueller Hinton broth was added to the wells labeled B-H. The crude extract and broth in well-A were mixed thoroughly before transferring 100 µL of the resultant mixture to well B. The same procedure was repeated for mixtures in wells B-H; finally, 100 µL was discarded from well H. Then 10 µL of microbial suspension pre-adjusted at 0.5 McFarland Standard was added to each well. All plates were incubated at 37°C for 24 hours. For this test, small volume of 96-well microtiter plates was used (microdilution). The procedure involves preparing a stock of 50 mg/mL of extract in DMSO (32).
To determine the MIC of each extract, 100 μL of Mueller Hinton broth was transferred to each well of the microtiter plate. From each extract, 100 μL was placed into wells of column 1. Using the pipette, the contents of each well were mixed thoroughly; 100 μL from column 1 was added to column 2, two-fold serial dilution was carried out throughout column 10 of which 100 μL was discarded. Then each well is inoculated with 50 μL of each strain of microorganism pre-adjusted at 0.5 McFarland Standard. All plates were incubated at 37°C for 24 hours.
3.7. Minimum Bactericidal Concentration (MBC)
For determination of MBC, samples from wells yielding negative microbial growth were subcultured on agar plates to determine the surviving cells after 24 hours of incubation.
4. Results
4.1. Specimen and Sex Determination
The biometric data of samples are shown in Table 1.
Biometric Data of the E. mathaei
| Weights (g) | Width (cm) | Height (cm) | ||||
|---|---|---|---|---|---|---|
| Aristotle’s Lantern | Spines | Gonads | Test | Total Weight | ||
| 3.86 ± 0.53 | 21.98 ± 3.86 | 3.51 ± 0.43 | 21.69 ± 5.70 | 82.21 ± 10.94 | 5.50 ± 0.75 | 2.97 ± 0.31 |
4.2. Antibacterial Activity
In this study, three solvents were used to extract and screen E. mathaei gut, gonad, and tests for antimicrobial activity against 4 bacterial species namely E. coli, P. aeruginosa, S. aureus, B. subtilis and two fungi species, including C. albicans and A. niger by the well agar diffusion method.
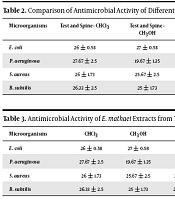
As shown in Table 2, all extracts of E. mathaei with 50 mg/mL concentration exhibited in vitro antimicrobial activity. The antimicrobial activity against all test microorganisms was present mainly in test extract. Antibacterial activity of the E. mathaei extracts varied with the different parts of the body, solvent used, and bacterial strain. Moreover, the data in this table indicate that test, and spines were more effective compared to other sea urchin studied.
Comparison of Antimicrobial Activity of Different Organs of E. mathaei (mm)
| Microorganisms | Test and Spine - CHCl3 | Test and Spine - CH3OH | Test and Spine n-hexane | Gonad - CHCl3 | Gonad - CH3OH | Gonad - n-Hexane | Gut - CHCl3 | Gut - CH3OH | Gut - n-Hexane |
|---|---|---|---|---|---|---|---|---|---|
| E. coli | 26 ± 0.58 | 27 ± 0.58 | 21 ± 2.31 | 17.33 ± 0.19 | 16.33 ± 0.19 | 14.66 ± 1.35 | 19.67 ± 1.35 | 19.33 ± 2.05 | 14.67 ± 0.19 |
| P. aeruginosa | 27.67 ± 2.5 | 19.67 ± 1.35 | 6.33 ± 2.5 | 13.67 ± 0.77 | 14.67 ± 0.19 | 6.67 ± 2.5 | 6.33 ± 1.35 | 12.33 ± 3.67 | 15.67 ± 1.35 |
| S. aureus | 26 ± 1.73 | 25.67 ± 2.5 | 28.67 ± 2.5 | 11 ± 2.31 | 15.67 ± 2.5 | 6.33 ± 1.35 | 4.33 ± 0.77 | 2.67 ± 0.19 | 9.33 ± 0.77 |
| B. subtilis | 26.33 ± 2.5 | 25 ± 1.73 | 26.67 ± 1.35 | 17 ± 0.58 | 12 ± 2.31 | 14 ± 0.58 | 8.33 ± 2.5 | 10.33 ± 0.19 | 9 ± 058 |
Antimicrobial Activity of E. mathaei Extracts from Test and Spines Organism (mm)
| Microorganisms | CHCl3 | CH3OH | n-Hexane | Tetracycline | Penicillin | Amoxicillin | Ceftriaxone | Gentamicin | Vancomycin |
|---|---|---|---|---|---|---|---|---|---|
| E. coli | 26 ± 0.58 | 27 ± 0.58 | 21 ± 2.31 | 13 ± 1.2 | 17 ± 1.01 | 17.33 ± 1.5 | 25.32 ± 1.7 | 28.33 ± 1.33 | 19.67 ± 1.35 |
| P. aeruginosa | 27.67 ± 2.5 | 19.67 ± 1.35 | 6.33 ± 2.5 | 18.6 ± 1.25 | 8.41 ± 1.04 | 5.31 ± 1.21 | 13.67 ± 1.33 | 18.31 ± 1.21 | 18.7 ± 1.25 |
| S. aureus | 26 ± 1.73 | 25.67 ± 2.5 | 28.67 ± 2.5 | 18.6 ± 1.33 | 7.37 ± 1.43 | 6.45 ± 1 | 8.33 ± 1.22 | 18.42 ± 1.04 | 10.33 ± 1.23 |
| B. subtilis | 26.33 ± 2.5 | 25 ± 1.73 | 26.67 ± 1.35 | 25.33 ± 1.11 | 19.12 ± 1.21 | 21.39 ± 1.02 | 35.51 ± 1.13 | 23.12 ± 1.35 | 16.46 ± 1.35 |
Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal (MBC) of test and Spines of E. mathaei Test Microorganisms (mg/mL)
| Extracts | Microorganisms | |||||||
|---|---|---|---|---|---|---|---|---|
| E. coli | P. aeruginosa | S. aureus | B. subtilis | |||||
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| CHCl3 | 12.5 | 50 | 12.5 | 25 | 25 | 50 | 12.5 | 50 |
| CH3OH | 12.5 | - | 25 | 25 | 25 | 25 | 12.5 | 50 |
| n-Hexane | 12.5 | 50 | 12.5 | 25 | 12.5 | 50 | 3.12 | 50 |
Shell and spine extracts of E. mathaei were more effective against pathogenic bacteria. The minimal inhibitory concentration of shell and spine hexane extract inhibited the growth of B. subtilis at 3.12 mg/mL (Tables 3 and 4).
5. Discussion
In most of the species studied, the whole bodies or body walls were tested in terms of the activity (24), but the present study aimed to evaluate the antimicrobial effects of various extracts in the different tissues of sea urchin E. mathaei on human pathogenic microorganisms. It was found that the extracts of the sea urchin E. mathaei exhibited antimicrobial activity, particularly the extracts of the gut, gonads, test and spines. Differences between active extracts indicated that several different compounds could be responsible for antimicrobial activity. Isolation and purification of the constituent active compounds are necessary to identify their chemical nature and to evaluate their potential as novel drugs (18).
Since the extraction was conducted by three different solvents, each one had a different polarity (i.e. nonpolar, semipolar, and bipolar), and regarding the fact that each solvent extract compound of a similar polarity, a close examination of the extracts would yield the existing compounds in the tissues under study. It is said, as hexane is a non-polar and chloroform is a semipolar solvent, it can be suggested that the antibacterial compounds in test and spines are non-polar or semipolar.
All extracts of the sea urchin E. mathaei exhibited in vitro antimicrobial activity. As shown in Table 2, the antibacterial activity of chloroform and methanolic extracts of test and spines against Gram-negative bacteria proved more effective compared with Gram-positive ones. However, as for the antibacterial activity of the hexane extract of test and spines, the effect against Gram-positive bacteria was more effective. The gut extracts exhibited higher antibacterial activity against Gram-negative bacteria.
5.1. Escherichia coli
The chloroform and methanolic extracts of test and spines against E. coli show more activities than other extracts. Also, these extracts were more effective against E. coli compared with penicillin, amoxicillin, tetracycline, and vancomycin.
5.2. Pseudomonas aeruginosa
The chloroform and methanolic extracts of test and spines against P. aeruginosa show more activities than other extracts. Also, these extracts were more effective against P. aeruginosa compared with penicillin, amoxicillin, tetracycline, vancomycin, gentamycin, and ceftriaxone.
5.3. Staphylococcus aureus
The n-hexane extracts of test and spines against S. aureus show more activities than other extracts. Also, these extracts were more effective against S. aureus compared with penicillin, amoxicillin, tetracycline, vancomycin, gentamycin, and ceftriaxone. In addition, Gonads were more effective than penicillin, amoxicillin, vancomycin, and ceftriaxone.
5.4. Bacillus subtilis
The methanolic extract of spines against B. subtilis shows more activities than other extracts. Also, this extract was more effective against B. subtilis compared only with vancomycin.
5.5. Conclusions
The results clearly showed the high antimicrobial activity of test and spines of the Persian Gulf sea urchin extracts against various Gram-positive and Gram-negative bacteria.
Acknowledgements
References
-
1.
Swathi V, Pratap PR, Monila N, Harshini S, RajaSekhar J, Ramesh A. The oceans-unlocking the treasured drugs. Int J Pharmaceut Chem Sci. 2012;1(3):1447-54.
-
2.
Proksch PP, Muller WEG. Frontiers in marine biotechnology. Horizon Bioscience; 2006.
-
3.
Perez MJ, Falque E, Dominguez H. Antimicrobial action of compounds from marine seaweed. Mar Drugs. 2016;14(3). [PubMed ID: 27005637]. [PubMed Central ID: PMC4820306]. https://doi.org/10.3390/md14030052.
-
4.
Carballeira NM, Cruz C, Sostre A. Identification of the novel 7-methyl-6-octadecenoic acid in Holothuria mexicana. J Nat Prod. 1996;59(11):1076-8. [PubMed ID: 8946750]. https://doi.org/10.1021/np9605091.
-
5.
Arizza V, Giaramita FT, Parrinello D, Cammarata M, Parrinello N. Cell cooperation in coelomocyte cytotoxic activity of Paracentrotus lividus coelomocytes. Comp Biochem Physiol A Mol Integr Physiol. 2007;147(2):389-94. [PubMed ID: 17329136]. https://doi.org/10.1016/j.cbpa.2007.01.022.
-
6.
Kuwahara R, Hatate H, Yuki T, Murata H, Tanaka R, Hama Y. Antioxidant property of polyhydroxylated naphthoquinone pigments from shells of purple sea urchin Anthocidaris crassispina. LWT - Food Sci Tech. 2009;42(7):1296-300. https://doi.org/10.1016/j.lwt.2009.02.020.
-
7.
Amarowicz R, Synowiecki J, Shahidi F. Sephadex LH-20 separation of pigments from shells of red sea urchin (Strongylocentrotus franciscanus). Food Chemistry. 1994;51(2):227-9. https://doi.org/10.1016/0308-8146(94)90262-3.
-
8.
McDermott F, Mattey DP, Hawkesworth C. Centennial-scale Holocene climate variability revealed by a high-resolution speleothem delta 18O record from SW Ireland. Science. 2001;294(5545):1328-31. [PubMed ID: 11701925]. https://doi.org/10.1126/science.1063678.
-
9.
Haug T, Kjuul AK, Styrvold OB, Sandsdalen E, Olsen OM, Stensvag K. Antibacterial activity in Strongylocentrotus droebachiensis (Echinoidea), Cucumaria frondosa (Holothuroidea), and Asterias rubens (Asteroidea). J Invertebr Pathol. 2002;81(2):94-102. [PubMed ID: 12445793].
-
10.
Rinehart KL, Shaw PD, Shield LS, Gloer JB, Harbour GC, Koker MES, et al. Marine natural products as sources of antiviral, antimicrobial, and antineoplastic agents. Pure Appl Chem. 1981;53(4):795-817. https://doi.org/10.1351/pac198153040795.
-
11.
Bryan PJ, McClintock JB, Watts SA, Marion KR, Hopkins TS. Antimicrobial activity of ethanolic body-wall extracts of echinoderms from the northern Gulf of Mexico. In: David B, Guille A, Feral JP, Roux M, editors. Echinoderms through time. Balkema: Rotterdam; 1994. p. 17-23.
-
12.
Anderson HA, Mathieson JW, Thomson RH. Distribution of spinochrome pigments in echinoids. Comp Biochem Physiol. 1969;28(1):333-45. https://doi.org/10.1016/0010-406x(69)91347-4.
-
13.
Iorizzi M, Bryan P, McClintock J, Minale L, Palagiano E, Maurelli S, et al. Chemical and biological investigation of the polar constituents of the starfish Luidia clathrata, collected in the Gulf of Mexico. J Nat Prod. 1995;58(5):653-71. [PubMed ID: 7623045].
-
14.
Canicatti C, Roch P. Studies onHolothuria polii (Echinodermata) antibacterial proteins. I. Evidence for and activity of a coelomocyte lysozyme. Experientia. 1989;45(8):756-9. https://doi.org/10.1007/bf01974579.
-
15.
Stabili L, Pagliara P. Antibacterial protection in Marthasterias glacialis eggs: Characterization of lysozyme-like activity. Comp Biochem Physiol B Biochem Mol Biol. 1994;109(4):709-13. https://doi.org/10.1016/0305-0491(94)90134-1.
-
16.
Leonard LA, Strandberg JD, Winkelstein JA. Complement-like activity in the sea star, Asterias forbesi. Dev Comp Immunol. 1990;14(1):19-30. [PubMed ID: 2338154].
-
17.
Beauregard KA, Truong NT, Zhang H, Lin W, Beck G. The detection and isolation of a novel antimicrobial peptide from the echinoderm, Cucumaria frondosa. Adv Exp Med Biol. 2001;484:55-62. [PubMed ID: 11419006]. https://doi.org/10.1007/978-1-4615-1291-2_5.
-
18.
Abubakar LA, Mwangi CM, Uku JU, Ndirangu SN. Antimicrobial activity of various extracts of the sea urchin Tripneustes gratilla (Echinoidea). African J Pharmacol Therapeut. 2012;1(1).
-
19.
Andersson L, Lidgren G, Bohlin L, Magni L, Ogren S, Afzelius L. Studies of Swedish marine organisms. I. Screening of biological activity. Acta pharmaceutica suecica. 1983;20(6):401-14.
-
20.
Andersson L, Bohlin L, Iorizzi M, Riccio R, Minale L, Moreno-Lopez W. Biological activity of saponins and saponin-like compounds from starfish and brittle-stars. Toxicon. 1989;27(2):179-88. [PubMed ID: 2718189]. https://doi.org/10.1016/0041-0101(89)90131-1.
-
21.
Ridzwan BH, Kaswandi MA, Azman Y, Fuad M. Screening for antibacterial agents in three species of sea cucumbers from coastal areas of Sabah. General Pharmacology: The Vascular System. 1995;26(7):1539-43. https://doi.org/10.1016/0306-3623(95)00041-0.
-
22.
Anbukkarasu S, Subramanian B, Elayaperumal N, Masilamani M. Antimicrobial properties of partially purified extract of starfish Luidia maculata. Int J Nat Prod Res. 2014;4(4):100-4.
-
23.
Shankarlal S, Prabu K, Natarajan E. Antimicrobial and antioxidant activity of purple sea urchin shell (Salmacis virgulata L. Agassiz and Desor 1846). Am-Euras J Sci Res. 2011;6(3):178-81.
-
24.
Uma B, Parvathavarthini R. Antibacterial effect of hexane extract of sea urchin, Temnopleurus alexandri (Bell, 1884). Int J PharmTech Res. 2010;2(3):1677-80.
-
25.
Benkendorff K, Davis AR, Bremner JB. Chemical defense in the egg masses of benthic invertebrates: An assessment of antibacterial activity in 39 mollusks and 4 polychaetes. J Invertebr Pathol. 2001;78(2):109-18. [PubMed ID: 11812113]. https://doi.org/10.1006/jipa.2001.5047.
-
26.
Prokof'eva NG, Chaikina EL, Kicha AA, Ivanchina NV. Biological activities of steroid glycosides from starfish. Comp Biochem Physiol B Biochem Mol Biol. 2003;134(4):695-701. https://doi.org/10.1016/s1096-4959(03)00029-0.
-
27.
Service M, Wardlaw AC. Echinochrome-A as a bactericidal substance in the coelomic fluid of Echinus esculentus (L.). Comp Biochem Physiol B Biochem Mol Biol. 1984;79(2):161-5. https://doi.org/10.1016/0305-0491(84)90008-7.
-
28.
Li C, Haug T, Moe MK, Styrvold OB, Stensvag K. Centrocins: Isolation and characterization of novel dimeric antimicrobial peptides from the green sea urchin, Strongylocentrotus droebachiensis. Dev Comp Immunol. 2010;34(9):959-68. [PubMed ID: 20438753]. https://doi.org/10.1016/j.dci.2010.04.004.
-
29.
Kazemi S, Heidari B, Rassa M. Antibacterial and hemolytic effects of aqueous and organic extracts from different tissues of sea urchin Echinometra mathaei on pathogenic streptococci. Int Aquat Res. 2016;8(4):299-308. https://doi.org/10.1007/s40071-016-0143-0.
-
30.
Price ARG. Studies on the echinoderm fauna of the western Arabian Gulf. J Nat Hist. 2010;15(1):1-15. https://doi.org/10.1080/00222938100770011.
-
31.
Kiani N, Heidari B, Rassa M, Kadkhodazadeh M, Heidari B. Antibacterial activity of the body wall extracts of sea cucumber (Invertebrata; Echinodermata) on infectious oral streptococci. J Basic Clin Physiol Pharmacol. 2014:1-7. [PubMed ID: 24468613]. https://doi.org/10.1515/jbcpp-2013-0010.
-
32.
Balouiri M, Sadiki M, Ibnsouda SK. Methods for in vitro evaluating antimicrobial activity: A review. J Pharm Anal. 2016;6(2):71-9. [PubMed ID: 29403965]. [PubMed Central ID: PMC5762448]. https://doi.org/10.1016/j.jpha.2015.11.005.