1. Background
There has been a lot of effort to identify natural products that may decrease the effects of carcinogenic substances in the environment. Honey is being used in traditional medicine for centuries. Humans evidently began to use honey dating back to 8000 years as shown by cave pictures (1). It has been therapeutically used by ancient Asian and European nations against wounds and gastrointestinal diseases (2). Furthermore, during the past few decades, honey played an important role in the indigenous medicine and has been used in laboratory and clinical studies. The most notable reports were on its antibacterial activities (3-6). Moreover, honey is widely recognized for its skin injuries healing. It provides a decreased dehydration environment that promotes wound healing. However, it is in a sticky fluid shape and topically is used to protect against infections.
The antibacterial and tissue repair activities of honey are related to its low acidity and release of the least amount of hydrogen peroxide (7, 8). The recent clinical case studies indicate that honey actively involves in the treatment of cutaneous infections (9).
The exact components of honey depend on bee feeding from different botanical sources.
In addition, honey samples contain different compositions of flavonoids and phenolic acids that synergistically act as antioxidants (6, 10-13).
Honey has been reported to have immunomodulatory activities (14) and is able to repair wound tissues by modulating the activity of monocyte cells and releasing anti-inflammatory cytokines (15). The role of honey in the treatment of rats bowel inflammation was investigated (16). The anti-inflammatory effects are due to a depletion in plasma concentrations of thromboxane B2, prostaglandin E2 (PGE2), and PGF2α, as well as the synergic effect of the phenolic compounds identified in the honey (17, 18).
The in vitro studies indicate that honey participates in cardiovascular protection by inhibiting the low-density lipoprotein oxidation and scavenging the generated reactive oxygen species (19-22).
Today, the cancer incidence is one of the human problems on which, excessive research has been performed to prevent the tumor growth. Scientists have considered skin cancer and attempted to treat skin tumors by using natural compounds (23).
So far, most of the studies investigated the potential benefits of honey in the general human health, but its efficacy on skin tumor treatment is not completely clear. It is reported that skin application of honey had an essential role in the treatment of radiation mucositis (24). In addition, honey has an antineoplastic activity in human bladder cancer cell lines and considerably is effective against implanted tumor growth in mice (25, 26).
According to these studies, it seems that honey acts through different mechanisms to inhibit the tumorigenesis potential. Recently, we have shown that during pregnancy, the body iron level plays an essential role in skin tumor incidence (27). Moreover, in another study, we showed the inhibitory effects of tannic acid on 7,12-dimethylbenz(a)anthracene (DMBA)-initiated mice skin carcinogenesis (28).
Thereby, the antioxidant property of honey may chelate the generated oxidant and decrease skin tumors. Hence, this study focused on the effect of honey on DMBA and croton oil-induced mice skin tumorigenesis.
2. Methods
2.1. Chemicals
The hair-removing cream was purchased from Tolidaro, Iran. Croton oil and DMBA were procured from Sigma (St. Louis, MO) Chemical Co., USA. [3H]-thymidine (specific activity of 82 Ci/mmol) was obtained from Amersham Corporation, UK. All other chemicals and biochemicals used in this study were either of analytical grade or of the highest purity grade available commercially.
2.2. Honey Sample
Floral honey was obtained from an authorized apiary at Oskou Agriculture Department situated in East Azerbaijan, Iran. Honey was sterilized by gamma-irradiation (25 kGy) and kept at room temperature away from direct sunlight.
2.3. Animals
In this study, Swiss albino female mice were obtained from the Central Animal House of Tabriz University of Medical Sciences. The animals were kept in polypropylene cages. For chronic (tumorigenesis) experiments, 20 mice and for acute experiments, six mice were housed in each group. They had a free access to a pellet diet and water ad libitum. The animals were kept at room temperature and exposed to 12-hour light and darkness cycles. The dorsal skin of the mice was shaved 48 hours before the treatment. Only those mice that did not show hair re-growth were used. The experiments were carried out in accordance with the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, National Academy Press, Washington, D.C. 1996).
2.4. Treatment of Animals and Tumorigenesis Studies
A total of 80 female Swiss mice were divided into four groups, 20 mice per group. Group I served as a positive control. Groups II, III, and IV were pretreated topically with honey dose-dependently at 100, 200, and 300 mg/mouse for two weeks. Six hours after the treatment with honey, the animals of all the four groups received a single topical application of DMBA (40 μg/100 μL acetone/mouse). One week after the application of DMBA, these animals were given a twice a week application of croton oil (0.5 mg/200 μL acetone/mouse) for a period of 30 weeks. The criteria for the diagnoses of tumors were the same as described by O'Connell et al. (29).
The mice were monitored for the incidence of tumors, which were recorded and plotted as a function of weeks on the test. The number of tumors per mouse and percentage of tumorigenesis incidence were recorded.
For studying the effect of honey on [3H]-thymidine incorporation into cutaneous DNA, according to the experimental protocol, 24 mice were divided into four groups (6 in each group) while group I served as the saline control. Groups II and IV were pretreated topically with honey (300 mg/mouse) for two weeks. After 3 hours, the animals of groups III and IV were treated with a single topical application of croton oil. After 18 hours, all the animals of the four groups were given an i.p. injection of [3H]-thymidine (15.0 μCi per animal per 0.2 mL saline). After 2 hours, all animals were sacrificed by cervical dislocation. Their skin tissues were removed, cleaned free of extraneous material, and processed for DNA separation.
2.5. [3H]-Thymidine Incorporation Assay
The incorporation of [3H]-thymidine in the epidermal DNA was done according to the method described by Smart et al. (30). Briefly, for obtaining clean epidermis, the skin was cleaned and a 10% homogenate (w/v) of skin tissue was prepared in ice-cold water containing an equal volume of ice-cold TCA (10%). The precipitate was washed with 5% of cold TCA and incubated with 10% of cold perchloric acid at 4ºC for 8 hours. After centrifugation, the precipitate was washed with 5% cold perchloric acid. The precipitate was dissolved in warm 10% perchloric acid followed by incubation in a boiling water bath for 30 minutes and then filtered through a Whatman 50 paper filter. The filtrate was counted for [3H]-thymidine. For counting in the sample, a fixed volume of this solution was added to the scintillation counter (LKB-Wallace-1410). The amount of DNA in the filtrate was estimated by the diphenylamine method of Giles and Myers and expressed as [3H] DPM/mg DNA (31).
2.6. Statistical Analysis
The Dunnett's t-test was used to obtain the level of significance between different groups followed by the analysis of variance test. The level of significance was set at P < 0.05.
3. Results
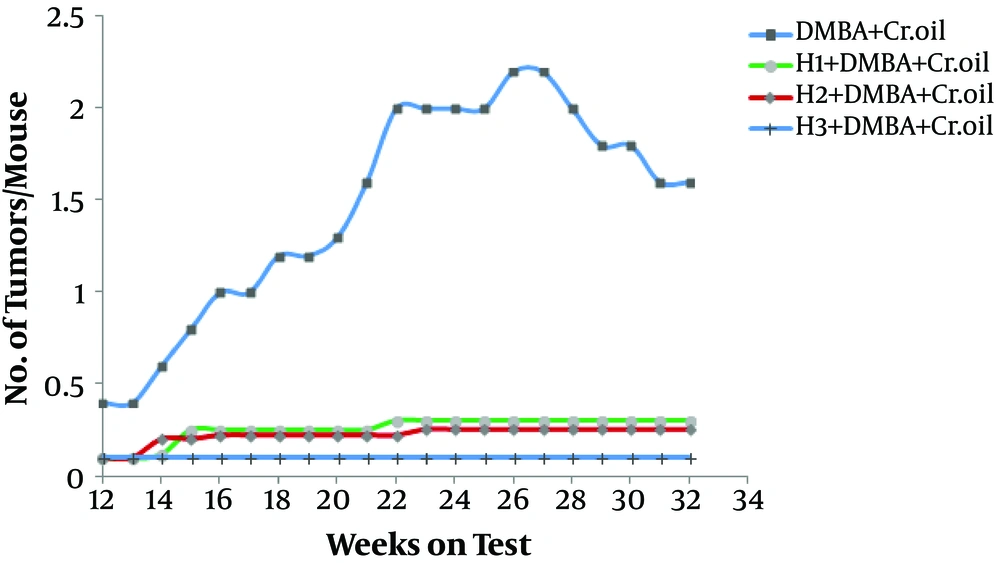
The effects of topical pretreatment with honey on the number of tumor and tumor incidences are shown in Figures 1 and 2, respectively. Figure 1 shows that the tumors significantly reduced in honey-pretreated animals as compared to the untreated positive control group.
Effect of honey on the yield of cutaneous tumors developed because of DMBA initiation and croton oil promotion in mice. The tumors were counted every week and are represented as the number of tumors per mouse plotted as a function of weeks on the test. Each value represents the mean number of tumors per mouse.
As shown in Figure 1, by week 22, the tumor yield in the DMBA-initiated and croton oil-promoted animals was 2/mouse while at the same time, in honey treated animals, there was more than an eight-fold reduction in the tumor number and the observed data were lest then 0.30 tumors/mouse. However, there were no tumor incidences in the corresponding groups of animals receiving only honey, acetone, DMBA, and croton oil alone.
Therefore, the tumor incidence was zero in the animals that received only acetone, DMBA, croton oil, and honey alone. That is, these animals did not develop any tumors during the course of the experiment.
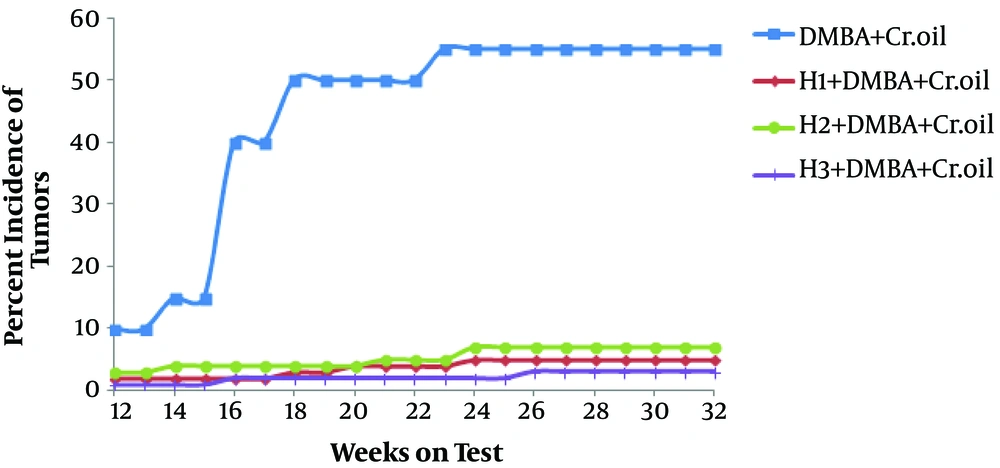
In a similar way, the effect of honey on the percentage of mice skin tumor is shown in Figure 2. By week 23, in DMBA and croton oil-treated animals, the tumor incidence was up to 50%; however, at this moment, the incidence was about 5% among honey-pretreated animals.
The effect of honey on [3H]-thymidine incorporation into cutaneous DNA is shown in Figure 3. As compared to the saline control group, about a three-fold increase in [3H]-thymidine incorporation in croton oil-treated animals was observed, while the application of honey significantly decreased this effect.
4. Discussion
The present study provides the first evidence that a topical application of honey inhibits the carcinogenic effects of DMBA and croton oil on mice skin.
Chemoprevention, which uses pharmacological agents with appropriate pharmacological properties (32, 33) or dietary materials, has been utilized in different forms such as macronutrients, micronutrients, and various phytochemicals (34-36). Recently, dietary manipulations, which affect the development of cancer, have been highlighted. Natural honey is rich in potent antioxidants, especially various polyphenols (37).
Studies have also been conducted to examine the effect of diet on the cause and augmentation of cancer. Results of some epidemiological investigations have also remarked that diet is an important risk factor for many common cancers (38). To date, a lot of natural antimutagenic chemical compounds with different chemical structures have been identified in human diet (39).
However, the inhibitory mechanisms in these studies appear to be due to the suppression of polycyclic aromatic hydrocarbons (PAHs) xenobiotic-metabolizing enzymes and finally, forming a stable bound with DNA (40).
In the present study, the detected reduction in the tumor incidence and tumor yield in honey-pretreated animals before DMBA suggested that honey might also function as a potent inhibitor of DMBA metabolites at the initiation stage so that the removal of generated peroxy radical, which participated in the production of DMBA metabolite, may play an essential role in the reduction of skin tumorigenesis.
Moreover, the decreased level of [3H]-thymidine incorporation into cutaneous DNA among honey pretreated mice suggests that binding some chemical compounds of natural honey to DNA plays an essential role in the DMBA and croton oil-induced skin tumorigenesis.
In conclusion, we suggest that honey as an effective natural preventive agent may provide protection against skin cancer.

![Effect of Honey on the inhibition of [<sup>3</sup>H]-thymidine incorporation into cutaneous DNA in mice. Exact treatment protocol and other experimental details are given in the text. Each value represents the mean ± SD of six animals (P < 0.05 when compared to saline-treated control mice). Effect of Honey on the inhibition of [<sup>3</sup>H]-thymidine incorporation into cutaneous DNA in mice. Exact treatment protocol and other experimental details are given in the text. Each value represents the mean ± SD of six animals (P < 0.05 when compared to saline-treated control mice).](https://services.brieflands.com/cdn/serve/3170b/f150d17dd0e3196a139f06e4ce1c04e6442b5849/jjnpp-In_Press-In_Press-57992-i003-preview.webp)