Abstract
Background:
Microemulsion (ME) is a method that has been developed in the recent years for synthesizing the nano-sized drug, metal or non-metal particles. These systems offer various advantages for drug delivery, such as ease of preparation, complete stability, and high drug solvability in many pharmaceutical applications.Objectives:
This study aimed at evaluating the protective effect of sour cherry kernel extract microemulsion on methimazole-induced nephrotoxicity in mice.Methods:
Sixty‐four male Swiss albino mice were divided to eight groups; each group consisted of eight mice. Group 1 was served as control, received normal saline; group 2 received base of ME without extract for 10 days; group 3 received a single dose of methimazole (100 mg/kg, ip) as positive control only on the 10th day; groups 4 to 6 received ME extract orally in doses of 250, 500, and 1000 mg/kg, respectively, during 10 days and methimazole (100 mg/kg, ip) on the 10th day; group 7 and 8 received ME extract and extract in dose of 1000 mg/kg for 10 days, respectively. Then, on the 11th day, serum samples were collected and used to determine the levels of serum creatinine (Cr) and blood urea nitrogen (BUN). After removing the kidney, malondialdehyde (MDA) and glutathione (GSH) levels and glutathione peroxidase (GPx) and superoxide dismutase (SOD) activity were also determined.Results:
The results obtained from the present study indicated a significant increase in the levels of Cr, BUN and MDA, and decrease of GSH, GPx and SOD by methimazole administration. Pre-treatment with ME extract showed reduction in the levels of Cr, BUN, MDA, and increase of GSH, GPx, and SOD. In addition, these observations were confirmed by histopathological changes of the kidney.Conclusions:
The current study showed that the extract had a protective effect on methimazole-induced nephrotoxicity and this effect was more for ME extract.Keywords
Methimazole Prunus cerasus L. Microemulsion Nephrotoxicity Mice
1. Background
In the recent years, nanotechnology-based drug delivery systems have progressed and due to the physicochemical properties of various drugs, more intelligent drug delivery systems are needed (1). Microemulsions (MEs) are modern colloidal drug vehicle systems. They are stable and isotropic mixtures of oil, water, surfactant and a co-surfactant with a diameter of 20 to 200 nm. These systems have various properties, such as optical transparency, complete stability, and high drug solvability in many pharmaceutical applications (2). Methimazole (MMI) is an anti-thyroid drug used for the treatment of hyperthyroidism associated with grave's disease (3). It has been well-known that MMI causes adverse effects, such as skin inflammation, allergies, pharyngitis, liver cirrhosis, and nephritis when using this drug to treat hyperthyroidism (4). The MMI-related nephrotoxicity is associated with obvious changes of function kidney and a high potential for decreased antioxidant capacity (5). Antioxidant compounds play a major role in various diseases. Early sources of naturally occurring antioxidants are grains, fruits, and vegetables (6). The medicinal plants are being used for valuable purposes around the world. They are important sources for the new drugs and health products (7). Prunus cerasus L. also, known as sour cherry, found in Europe and Southwestern Asia (8), has white flowers and tart red fruits (9). Sour cherry kernels include 32% to 36% of vegetable oils and 64% to 68% of the kernel is a solid deduction comprised of flavonoids, polyphenols, anthocyanins, stilbenes, and catechins (10).
2. Objectives
This study aimed at evaluating the protective effects of sour cherry (Prunus cerasus L.) kernel hydroalcoholic extract microemulsion on MMI-induced nephrotoxicity in mice.
3. Methods
3.1. Chemicals
Thiobarbituric acid (TBA), Trichloroacetic acid (TCA), reduced glutathione (GSH), 5, 5-dithiobis (2-nitrobenzoic acid) (DTNB), Coomassie blue G, tween-80, propylene glycol (PG), bovine serum albumin (BSA), Bradford reagent, paraffin, formalin, ethanol, and methimazole powder used for this experiment were obtained from Sigma. Sesame oil was obtained from Sabz Kooh, Iran. Ketamine and xylazine were purchased from Alfasan Co. (Holland).
3.2. Animals
Adult male Swiss albino mice (20 to 25 g, eight weeks old, Research Center and Animal House of Jundishapur University of Medical Sciences, Ahvaz, Iran) were kept in a polycarbonate cage under standard conditions (ambient temperature of 25 ± 2ºC) with a 12 hours light: 12 hours dark cycle and free access to water and food. The study protocols were approved by the Ethics Committee of Ahvaz Jundishapur University of Medical Sciences.
3.3. Extract Preparation
Sour cherry kernel seeds were dried and eliminated from the external shell components. The kernel was broken down and extracted with n-hexane during four hours. Accordingly, the oil and solid fraction were broke up. The solid fraction was soaked in 70% ethanol for three days. The extract was filtered and was kept in the freeze dryer for 48 hours (Virtis, USA) until they were dried and the solvent was evaporated (11).
3.4. Microemulsion Preparation
Pseudo-ternary phase diagram was made using the titration method to investigate the Microemulsion (ME) existence range. The ME system was made-up of sesame oil (oily phase), tween-80 as surfactant, and propylene glycol as co-surfactant. The proportion of surfactant to co-surfactant at 3:1 was used. The doses of sour cherry kernel extract (250, 500, and 1000 mg/kg) were added to this system, then a clear and transparent system was obtained. The MEs were kept at three temperatures (4ºC, 25ºC, and 37ºC) and checked for turbidity and coalescence. Furthermore, samples were centrifuged at 10000 rpm for 30 minutes at 25ºC. The components of appropriate formulation were as follows: 2.43% oil, 66.55% S+C; and 31.02% water. The microemulsions were appraised regarding their viscosity, particle size, and pH.
3.4.1. Viscosity Measurement
Viscosity (η) was measured with a Brookfield viscometer (DV-II + Pro) at 25ºC and 100 rpm (12).
3.4.2. Particle Size Measurement
Particle size analysis was measured at 25ºC by Scatter Scope 1 Quidix. Moreover, their refractory indices were also calculated (13).
3.4.3. The pH Determination
The pH of samples was measured on the basis of experiment at 25ºC, using a pH-meter (Mettler Toledo Seven Easy).
3.5. Experimental Design
In this study, animals were randomly allocated to eight experimental groups; each group consisted of eight mice. Group 1 as negative control group, received normal saline orally for 10 days; group 2 received base of ME without extract orally for 10 days, group 3 received a single dose of MMI (100 mg/kg intraperitoneally) as positive control (14) only on the 10th day; groups 4 to 6 received ME extract orally at doses of 250, 500, and 1000 mg/kg respectively, during 10 days and MMI (100 mg/kg intraperitoneally) on the 10th day. Groups 7 and 8 received ME extract and extract at dose of 1000 mg/kg for 10 days, respectively.
3.6. Sample Collection
On the 11th day, animals were anesthetized with ketamine and xylazine and blood samples were gathered. Serum was centrifuged at 3000 rpm for 10 minutes and stored at -20ºC until assayed. The kidney was removed and immersed in 10% buffered formalin solution for histological examination and small fragments of the kidney was frozen at -70ºC degree for measuring glutathione peroxidase (GPx) and superoxide dismutase (SOD) activity, and malondialdehyde (MDA) and glutathione (GSH) levels.
3.7. Serum Analysis
The levels of serum creatinine (Cr) and blood urea nitrogen (BUN) were measured for kidney function using the biochemical analyzer with respective test kits.
3.8. Malondialdehyde Levels Assay
Lipid peroxidation was measured by determining the amounts of MDA. Assays of MDA content were performed using the reaction of color TBA method (Buege and Aust) (15) involving the measurement of total MDA. The sample containing 0.5 mL of homogenized tissue was blended with 2.5 mL of TCA (10%, w/v) and centrifuged at 3000 rpm for 10 minutes. Then, 1 mL of TBA solution (0.67%, w/v) was added to 2 mL of each sample in a test tube. The solution was heated for 10 minutes in boiling water until developing a pink-colored solution. After cooling, the absorbance was recorded at 532 nm using a spectrophotometer. The results were declared as nmol/mg protein.
3.9. Glutathione Levels Assay
The levels of GSH were measured by the response with Ellman’s reagent (5-5-dithiobis 2-nitrobenzoic acid) to give a yellow colored complex. The absorbances were read at 412 nm (16). A portion of tissue homogenate was precipitated with 0.1 mL of 25% TCA and centrifuged at 1000 rpm for 10 minutes. The reference cuvette contained 2 mL of 0.5 mM DTNB in 0.2 M phosphate buffer (pH = 8) and 0.1 mL of the floating to a final volume of 3 mL, and the yellow color developed was evaluated in the spectrophotometer. The results were declared as nmol/mg protein.
3.10. Glutathione Peroxidase Activity Assay
The levels of GPx were evaluated with the GPx kit (Randox Labs, Crumlin, UK). It was declared as units/mg protein.
3.11. Superoxide Dismutase Activity Assay
The homogenate was centrifuged for 20 minutes at 4ºC, 12,000 × g, and the absorbance was evaluated in the spectrophotometer. The SOD activity was determined using the Martin method (17). The results were declared as U/mg protein.
3.12. Histopathological Assessments
The kidney was fixed in 10% buffered formalin. After one day, samples were immersed in 70% ethanol and processed for paraffin sectioning. The paraffin blocks were cut to pieces of 5 µm, which were then stained with hematoxylin and eosin to evaluate under the light microscope.
3.13. Statistical Analysis
The SPSS statistical software (SPSS for Windows, version 18.0) was used to analyze the data. The results were presented as mean ± SD and all statistical comparisons were obtained by means of one-way analysis of variance (ANOVA) test pursued by Tukey’s post hoc test. P < 0.05 was considered as statistically significant.
4. Results
4.1. Microemulsion Results
The ingredients of appropriate formulation were as follows: oil, 2.43%; surfactant/co-surfactant, 66.55%, and water, 31.02% (Figure 1). The ME formulations had mean viscosity of 316.6 ± 23.7 centipoises, the mean droplet sizes of 7.9 ± 1.1 nm, and mean pH of 4.8 ± 0.2.
The Pseudo-Ternary phase diagram of the oil-surfactant/co-surfactant mixture-water system at the 3:1 weight ratio of tween-80 propylene glycol at ambient temperature; dark area shows ME zone.

4.2. Effects of Extract and Methimazole on Creatinine and Blood Urea Nitrogen Levels
Results showed that MMI alone caused a significant increase in the levels of Cr (1.35 ± 0.32 mg/dL) and BUN (68.31 ± 6.71mg/dL) in comparison to the group receiving saline (0.66 ± 0.12 and 31.12 ± 5.05 respectively) (P < 0.001). Pre-treatment with ME extract in groups 4 to 6 showed a decrease in the levels of Cr and BUN at all doses compared with the group receiving MMI, yet it significantly decreased the levels of Cr at a dose of 1000 mg/kg (P < 0.05) and significantly decreased the levels of BUN at doses of 500 and 1000 mg/kg (P < 0.01 and P < 0.001 respectively) (Figure 2).
Effects of pre-treatment with ME extract on serum levels of Cr and BUN in MMI-induced nephrotoxicity. All values are expressed as mean ± SD, N = 8. *Significant difference in comparison with the control group (***P < 0.001). #Significant difference in comparison with the MMI group (#P < 0.05, ##P< 0.01, ###P < 0.001).

4.3. Effects of Extract and Methimazole on Malondialdehyde and Glutathione Levels
Results showed that the MDA and GSH of kidney tissue in the group receiving MMI were 2.3 ± 0.35 and 1.9 ± 0.4 nmol/mg protein and in the group receiving saline, these were 1.2 ± 0.21 and 5.2 ± 0.7nmol/mg protein. The MMI significantly increased the amount of MDA and decreased the amount of GSH in renal tissue in comparison with the group receiving saline (P < 0.001). Pre-treatment with ME extract in groups 4 to 6 decreased MMI-induced increase in MDA in renal tissue compared with the group receiving MMI, yet it was significantly decreased in MDA at doses of 500 and 1000 mg/kg (P < 0.05 and P < 0.001, respectively). Also, pre-treatment with ME extract in groups 4 to 6 increased MMI-induced decrease in GSH in renal tissue compared with the group receiving MMI, yet it was significantly increased in GSH at doses of 500 and 1000 mg/kg (P < 0.05) (Figure 3).
Effects of pre-treatment with ME extract on MDA and GSH levels in MMI-induced nephrotoxicity. All values are expressed as mean ± SD, N = 8. *Significant difference in comparison with the control group (***P < 0.001). #Significant difference in comparison with the MMI group (#P < 0.05, ###P < 0.001).

4.4. Effects of Extract and Methimazole on Glutathione Peroxidase and Superoxide Dismutase Activity
Results showed that the GPx and SOD activity in the group receiving MMI were 0.2 ± 0.05 and 0.55 ± 0.1 U/mg protein and in the group receiving saline, these were 0.5 ± 0.1 and 1.7 ± 0.27 U/mg protein. The MMI significantly decreased the activity of Gpx and SOD in renal tissue in comparison to the group receiving saline (P < 0.001). Pre-treatment with ME extract in groups 4 to 6 increased MMI-induced reduction of GPx activity in renal tissue compared with the group receiving MMI, yet it significantly increased GPx activity at dose of 1000 mg/kg (P < 0.05). Also, pre-treatment with ME extract in groups 4 to 6 increased MMI-induced reduction of SOD activity in renal tissue compared with the group receiving MMI, yet it was significantly increased in SOD at doses of 500 and 1000 mg/kg (P < 0.05 and P < 0.001, respectively) (Figure 4).
Effects of pre-treatment with ME extract on GPx and SOD activity in MMI-induced nephrotoxicity. All values are expressed as mean ± SD, N = 8. *Significant difference in comparison with the control group (***P < 0.001). #Significant difference in comparison with the MMI group (#P < 0.05, ###P < 0.001).

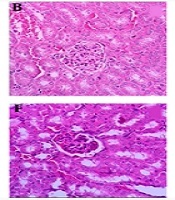
4.5. The Light Microscopic Findings
The histopathological analysis of renal tissues in the groups 1, 2, 7 and 8 showed a normal structure without significant pathologic changes. The kidney tissue of mice in group 3 (positive control) showed degenerative changes in glomeruli, hyaline deposition in the form of eosinophilic material in the tubules, some tubules with moderate to severe epithelial atrophic changes, widening of the lumen of tubules and foci of congestion of capillary loops. In the groups 4 to 6, increasing of dosage of ME from 250 mg/kg to 1000 mg/kg showed greater protection from destructive effects of MMI kidney tissue (Figure 5).
Histopathological observations (kidney sections stained with Hematoxylin & Eosin, magnification × 40) showing effects of ME extract on MMI-induced nephrotoxicity changes in mouse kidney. A, Normal; B, ME base treated group; C, MMI treated group; D, E and F are MMI group pre-treated with 250, 500 and 1000 mg/kg of ME extract, respectively; G, ME extract treated group; H, extract treated group.

5. Discussion
The kidney has a major role in the elimination of a wide diversity of xenobiotics. Besides, the renal system regulates the volume of blood and extracellular fluid, acid-base homeostasis, and electrolyte combination (18). Kidney injuries caused by drugs are common in humans and could disturb these functions (19).
Cr and BUN (20) are two evaluation indices for renal functions. Treatment with MMI causes nephrotoxicity, which has major negative side effects (3). Measuring blood levels of enzymes, biochemicals are important for indication of damage to any tissue. High levels of any factor may connote a fracture of active pathologies (21, 22). Also, in this study, increasing Cr and BUN levels in MMI-treated group demonstrated renal toxicity (P < 0.001), which may possibly be due to damage generated in kidney tubules in comparison to the control group. Lipid peroxidation is a common procedure of cellular damage, which is a common consequence of oxidant stress, owning toxic characteristics (23). The GSH is an α-amino acid and a tripeptide evolved as a molecule that protects cells against radical toxicity (24). Use of MMI could increase MDA levels in kidney tissue and reduce the level of GSH (P < 0.001). This represents the increase of oxidative stress and oxidative detriment to macro molecules, tissues or organs (25). GPx is an enzyme with peroxidase function, whose principal biological role is to defend cells from serious detriment (26) and SOD is a metallic enzyme and an important link in a biological defense mechanism, to be rapidly cleared by the kidneys (27). The results of this study showed the activities of antioxidants enzymes; GPx and SOD were significantly (P < 0.001) decreased in kidney tissues of MMI-treated group, in comparison to the control group, which demonstrated that MMI has caused severe oxidative stress.
In a study evaluating the effect of selenium on methimazole nephrotoxicity, antioxidant enzyme activities, superoxide dismutase, catalase, and glutathione peroxidase decreased. Lipid peroxidation indicated an increment by high kidney malondialdehyde levels. Methimazole-treated rat kidneys demonstrated leucocytic infiltrations, vascular congestion, and narrowed Bowman’s space. The observed renal protection effects of selenium can be attributed to the high content of antioxidant selenium (5). In another study, renal dysfunction and decrease in the size and length of the tubules was observed (25). The current results are in agreement with recent experiments.
It has been reported that sour cherry kernel has antioxidant compounds, such as flavonoids, polyphenols, anthocyanins, stilbenes, and catechins that work by donating electrons or hydrogen atoms to free radicals (28, 29), and lead to protection against oxidative stress. This property has led to the use of flavonoids (anthocyanins, catechins) in studies on oxidative stress, cancer, and aging processes (30). It has been reported that anthocyanins inhibit the growth of cancer cells. In some studies, it has also been shown that it prevents the spread of tumors and the growth of colon cancer cells in mice (31). In addition, cyanidin shows more anti-inflammatory effects than aspirin (32). Recent studies indicate that anthocyanins are a quality index for a cherry extract, and it has been shown that cherry extract reduces inflammation, edema, and the appearance of gout and arthritis pain (33).
Furthermore, the histological examinations of kidney confirmed the protective effects of extract against the MMI-induced kidney damage (34) that showed considerable improvement in proximal and distal convoluted tubules in pre-treated groups. A lot of studies have been done on the sour cherry kernel that evaluated several properties on the inflammation (35), systemic and dermal toxicity (36), ischemic-reperfused myocardium (37), and protective effects (38, 39). These results indicate that sour cherry kernel is safe and has no adverse effects.
Since this plant has a potent antioxidant and anti-inflammatory effects, the protective effect of the extract of this plant in MMI-induced nephrotoxicity was inspected in this experiment. Therefore, we studied different doses of ME extract to evaluate of oxidant/antioxidant parameters as markers of oxidative stress-induced kidney injury, and the best results were observed at the dose level of 1000 mg/kg.
Results showed group 2 that received only microemulsion base did not show any significant differences in comparison to the group receiving saline on all biochemical parameters and histopathological observations. as well as, between group 7 and 8 with control group did not have any significant differences. Therefore, it can be concluded that the extract and microemulsion base have no toxic effects. Using the data of this report and by observing no record of toxicity of extract, ME base and ME extract, bioavailability enhancement of bioflavonoids and antioxidant in sour cherry seed kernel extract and the purposeful drug delivery was indicated. Therefore, nano size range of particle showed a better efficacy and protective role of sour cherry kernel extract against oxidative damage.
Eventually, the findings of the present study demonstrated that ME of sour cherry (Prunus cerasus L.) kernel extract showed protective effects against MMI-induced nephrotoxicity in mice.
References
-
1.
Orive G, Hernández RM, Gascón AR, Pedraz JL. Micro and nano drug delivery systems in cancer therapy. Cancer Therapy. 2005;3(1):131-8.
-
2.
Heuschkel S, Goebel A, Neubert RH. Microemulsions-modern colloidal carrier for dermal and transdermal drug delivery. J Pharm Sci. 2008;97(2):603-31. [PubMed ID: 17696162]. https://doi.org/10.1002/jps.20995.
-
3.
Tashkandi BM, Saleh HA, Jambi HA. The protective effect of Vitamin E and selenium on methimazole-induced hepato-renal toxicity in adult rats. Life Sci J. 2014;11(10):894-9.
-
4.
Edward AC. Thyroid hormones and drugs that affect the thyroid. In: Smith CM, Reynard AM, editors. Text-book of pharmacology. Philadelphia: W.B. Saunders Company; 1992. p. 652-6.
-
5.
Ben Amara I, Troudi A, Garoui E, Hakim A, Boudawara T, Zeghal KM, et al. Protective effects of selenium on methimazole nephrotoxicity in adult rats and their offspring. Exp Toxicol Pathol. 2011;63(6):553-61. [PubMed ID: 20472414]. https://doi.org/10.1016/j.etp.2010.04.007.
-
6.
Mishra N, Dubey A, Mishra R, Barik N. Study on antioxidant activity of common dry fruits. Food Chem Toxicol. 2010;48(12):3316-20. [PubMed ID: 20804814]. https://doi.org/10.1016/j.fct.2010.08.029.
-
7.
Chen SL, Yu H, Luo HM, Wu Q, Li CF, Steinmetz A. Conservation and sustainable use of medicinal plants: problems, progress, and prospects. Chin Med. 2016;11:37. [PubMed ID: 27478496]. [PubMed Central ID: PMC4967523]. https://doi.org/10.1186/s13020-016-0108-7.
-
8.
Seeram NP, Momin RA, Nair MG, Bourquin LD. Cyclooxygenase inhibitory and antioxidant cyanidin glycosides in cherries and berries. Phytomedicine. 2001;8(5):362-9. [PubMed ID: 11695879]. https://doi.org/10.1078/0944-7113-00053.
-
9.
Piccolella S, Fiorentino A, Pacifico S, D'Abrosca B, Uzzo P, Monaco P. Antioxidant properties of sour cherries (Prunus cerasus L.): Role of colorless phytochemicals from the methanolic extract of ripe fruits. J Agric Food Chem. 2008;56(6):1928-35. [PubMed ID: 18303821]. https://doi.org/10.1021/jf0734727.
-
10.
Szabo ME, Gallyas E, Bak I, Rakotovao A, Boucher F, de Leiris J, et al. Heme oxygenase-1-related carbon monoxide and flavonoids in ischemic/reperfused rat retina. Invest Ophthalmol Vis Sci. 2004;45(10):3727-32. [PubMed ID: 15452083]. https://doi.org/10.1167/iovs.03-1324.
-
11.
Abass MA, Kabbashi AS, Garbi MI. In vitro antioxidant activity, phytochemical analysis and cytotoxicity of Ethanolic leaves extract of moringa oleifera lam. Int J Multidiscip Res Dev. 2015;2(11):73-7.
-
12.
Salimi A, Sharif Makhmal Zadeh B, Moghimipour E. Preparation and characterization of cyanocobalamin (vit B12) microemulsion properties and structure for topical and transdermal application. Iran J Basic Med Sci. 2013;16(7):865-72. [PubMed ID: 23997918]. [PubMed Central ID: PMC3758059].
-
13.
Moghimipour E, Salimi A, Leis F. Preparation and evaluation of tretinoin microemulsion based on pseudo-ternary phase diagram. Adv Pharm Bull. 2012;2(2):141-7. [PubMed ID: 24312785]. [PubMed Central ID: PMC3845984]. https://doi.org/10.5681/apb.2012.022.
-
14.
Heidari R, Babaei H, Roshangar L, Eghbal MA. effects of enzyme induction and/or glutathione depletion on methimazole-induced hepatotoxicity in mice and the protective role of N-acetylcysteine. Adv Pharm Bull. 2014;4(1):21-8. [PubMed ID: 24409405]. [PubMed Central ID: PMC3885364]. https://doi.org/10.5681/apb.2014.004.
-
15.
Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302-10. [PubMed ID: 672633]. https://doi.org/10.1016/S0076-6879(78)52032-6.
-
16.
Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82(1):70-7. [PubMed ID: 13650640]. https://doi.org/10.1016/0003-9861(59)90090-6.
-
17.
Martin JP Jr, Dailey M, Sugarman E. Negative and positive assays of superoxide dismutase based on hematoxylin autoxidation. Arch Biochem Biophys. 1987;255(2):329-36. [PubMed ID: 3036004]. https://doi.org/10.1016/0003-9861(87)90400-0.
-
18.
Kurts C, Panzer U, Anders HJ, Rees AJ. The immune system and kidney disease: basic concepts and clinical implications. Nat Rev Immunol. 2013;13(10):738-53. [PubMed ID: 24037418]. https://doi.org/10.1038/nri3523.
-
19.
Shirani M, Alizadeh S, Mahdavinia M, Dehghani MA. The ameliorative effect of quercetin on bisphenol A-induced toxicity in mitochondria isolated from rats. Environ Sci Pollut Res Int. 2019;26(8):7688-96. [PubMed ID: 30666577]. https://doi.org/10.1007/s11356-018-04119-5.
-
20.
Sacks CR, Peterson RA, Kimmel PL. Perception of illness and depression in chronic renal disease. Am J Kidney Dis. 1990;15(1):31-9. https://doi.org/10.1016/s0272-6386(12)80589-0.
-
21.
Sadeghi A, Kalantar M, Molavinia S, Houshmand G, Bahadoram M, Esmaeilizadeh M, et al. Ameliorative effects of hydroalcoholic extract of Lavandula officinalis L. on cyclophosphamide-induced nephrotoxicity in mice. J Nephropathol. 2017;6(4):324-32. https://doi.org/10.15171/jnp.2017.52.
-
22.
Fujii T. Toxicological correlation between changes in blood biochemical parameters and liver histopathological findings. J Toxicol Sci. 1997;22(3):161-83. [PubMed ID: 9279820]. https://doi.org/10.2131/jts.22.3_161.
-
23.
Pratico D, Basili S, Vieri M, Cordova C, Violi F, Fitzgerald GA. Chronic obstructive pulmonary disease is associated with an increase in urinary levels of isoprostane F2alpha-III, an index of oxidant stress. Am J Respir Crit Care Med. 1998;158(6):1709-14. [PubMed ID: 9847257]. https://doi.org/10.1164/ajrccm.158.6.9709066.
-
24.
Meister A. [1] Glutathione metabolism. Methods Enzymol. 1995;251:3-7. https://doi.org/10.1016/0076-6879(95)51106-7.
-
25.
Bradley SE, Bradley GP, Stéphan F. Role of structural imbalance in the pathogenesis of renal dysfunction in the hypothyroid rat. Trans Assoc Am Physicians. 1972;85:344-52. [PubMed ID: 4660014]. https://doi.org/10.7547/87507315-61-9-344.
-
26.
Ran Q, Liang H, Ikeno Y, Qi W, Prolla TA, Roberts LJ 2nd, et al. Reduction in glutathione peroxidase 4 increases life span through increased sensitivity to apoptosis. J Gerontol A Biol Sci Med Sci. 2007;62(9):932-42. [PubMed ID: 17895430]. https://doi.org/10.1093/gerona/62.9.932.
-
27.
Hussain T, Shukla GS, Chandra SV. Effects of cadmium on superoxide dismutase and lipid peroxidation in liver and kidney of growing rats: In vivo and in vitro studies. Pharmacol Toxicol. 1987;60(5):355-8. [PubMed ID: 3615346]. https://doi.org/10.1111/j.1600-0773.1987.tb01526.x.
-
28.
Seymour EM, Singer AA, Kirakosyan A, Urcuyo-Llanes DE, Kaufman PB, Bolling SF. Altered hyperlipidemia, hepatic steatosis, and hepatic peroxisome proliferator-activated receptors in rats with intake of tart cherry. J Med Food. 2008;11(2):252-9. [PubMed ID: 18598166]. https://doi.org/10.1089/jmf.2007.658.
-
29.
Kalantari H, Shamsi Ehsan T, Samimi A, Kheradmand P, Shirani M, Zeidooni L. Histopathological and biomedical parameters determination in the protective effect of hydroalcoholic extract of Allium jesdianum on hepatotoxicity induced by bromobenzene in mice. Iran J Pharm Sci. 2018;14(2):15-24.
-
30.
Collins AR. Antioxidant intervention as a route to cancer prevention. Eur J Cancer. 2005;41(13):1923-30. [PubMed ID: 16111883]. https://doi.org/10.1016/j.ejca.2005.06.004.
-
31.
Wang LS, Stoner GD. Anthocyanins and their role in cancer prevention. Cancer Lett. 2008;269(2):281-90. [PubMed ID: 18571839]. [PubMed Central ID: PMC2582525]. https://doi.org/10.1016/j.canlet.2008.05.020.
-
32.
Blando F, Gerardi C, Nicoletti I. Sour cherry (prunus cerasus l) anthocyanins as ingredients for functional foods. J Biomed Biotechnol. 2004;2004(5):253-8. [PubMed ID: 15577186]. [PubMed Central ID: PMC1082898]. https://doi.org/10.1155/S1110724304404136.
-
33.
Bell PG, McHugh MP, Stevenson E, Howatson G. The role of cherries in exercise and health. Scand J Med Sci Sports. 2014;24(3):477-90. [PubMed ID: 23710994]. https://doi.org/10.1111/sms.12085.
-
34.
Cano-Europa E, Blas-Valdivia V, Franco-Colin M, Gallardo-Casas CA, Ortiz-Butron R. Methimazole-induced hypothyroidism causes cellular damage in the spleen, heart, liver, lung and kidney. Acta Histochem. 2011;113(1):1-5. [PubMed ID: 19775732]. https://doi.org/10.1016/j.acthis.2009.07.004.
-
35.
Mahmoud F, Haines D, Al-Awadhi R, Dashti AA, Al-Awadhi A, Ibrahim B, et al. Sour cherry (Prunus cerasus) seed extract increases heme oxygenase-1 expression and decreases proinflammatory signaling in peripheral blood human leukocytes from rheumatoid arthritis patients. Int Immunopharmacol. 2014;20(1):188-96. [PubMed ID: 24631368]. https://doi.org/10.1016/j.intimp.2014.02.031.
-
36.
Bak I, Czompa A, Csepanyi E, Juhasz B, Kalantari H, Najm K, et al. Evaluation of systemic and dermal toxicity and dermal photoprotection by sour cherry kernels. Phytother Res. 2011;25(11):1714-20. [PubMed ID: 21751269]. https://doi.org/10.1002/ptr.3580.
-
37.
Czompa A, Gyongyosi A, Czegledi A, Csepanyi E, Bak I, Haines DD, et al. Cardioprotection afforded by sour cherry seed kernel: the role of heme oxygenase-1. J Cardiovasc Pharmacol. 2014;64(5):412-9. [PubMed ID: 24949584]. https://doi.org/10.1097/FJC.0000000000000132.
-
38.
Salimi A, Motaharitabar E, Goudarzi M, Rezaie A, Kalantari H. Toxicity evaluation of microemulsion (nano size) of sour cherry kernel extract for the oral bioavailability enhancement. Jundishapur J Nat Pharm Prod. 2014;9(1):16-23. [PubMed ID: 24644434]. [PubMed Central ID: PMC3957138]. https://doi.org/10.17795/jjnpp-14370.
-
39.
Moosavi M, Rezaei M, Kalantari H, Behfar A, Varnaseri G. l-carnitine protects rat hepatocytes from oxidative stress induced by T-2 toxin. Drug Chem Toxicol. 2016;39(4):445-50. [PubMed ID: 26888052]. https://doi.org/10.3109/01480545.2016.1141423.