1. Background
The most common neurodegenerative disorder is Alzheimer's disease (AD) that causes a progression from episodic memory impairment to a slow global cognitive decline. Biochemical dysfunctions in the AD include changes in amyloid precursor protein metabolism, tau phosphorylation, oxidative stress, energy metabolism and mitochondrial impairment, inflammation, membrane lipid dysregulation, and neurotransmitter pathway disruption (1). The hippocampal formation and the parahippocampal region are affected by the AD in the early stages of the disease (2). Atrophy, in particular, in the hippocampal subfields, prior to the development of cognitive impairment, occurs preferentially with amyloid β (Aβ) accumulation (3). The amyloid plaque deposition is the primary event that leads to inflammatory reaction, neurofibrillary tangles formation and finally, neuronal cell death (4), in particular, in the neocortex and limbic system (5). The new drug development against AD has been mainly focused on compounds that inhibit the effects of Aβ (6) that, unfortunately, have not shown positive results in human studies (7). However, the regulation of its production and clearance is not fully understood (8); thus, therapeutic strategies that can protect neurons and attenuate Aβ generation in the brain are desirable (5).
Scopolamine, a muscarinic receptor antagonist, is known to impair the performance of learning and memory by blocking cholinergic signaling and through indirect mechanisms (9). Scopolamine treatment increases Aβ deposition (10), oxidative stress activation (11), and synaptic dysfunction (12). It has been widely used as a pharmacological model of cognitive impairment in the AD to identify the potential therapeutic target molecules for AD treatment (12) and screening of anti-dementia drugs (13).
Nowadays, Ginkgo biloba extract has been widely used in some countries for cognitive disorders (e.g., memory loss with aging and AD) (14), and observational research suggests that it might prevent the AD (15). What is more, Ginkgo biloba leaves do not lead to obvious side effects within the recommended dose (16). Ginkgo biloba extract, as an antioxidant, prompts the release of neuroprotectants to inhibit the neuropathological processes of the AD including Aβ-induced neuronal cell death, Aβ oligomerization, and glial cell activation. Accordingly, Ginkgo biloba extract increases neurogenesis and suppresses the happening of pathological processes and cognitive decline (17).
2. Objectives
However, the protective effects of Ginkgo biloba extract on the number of congophilic amyloid plaques in the hippocampus and cingulate cortex have not yet been reported. Therefore, the present study aimed to evaluate the effects of short-term administration of Ginkgo biloba extract on the number of congophilic amyloid plaques in the hippocampus and cingulate cortex, as well as on hippocampal and cingulate cortex neuron numbers before and after scopolamine administration.
3. Methods
3.1. Animals
Adult male Wistar rats (200 ± 20 g) were purchased from the Pasteur Institute of Iran (Tehran). The rats were maintained at a constant temperature (22 ± 3ºC) with 12-hour-light/12-hour-dark cycle and they were given free access to standard food and tap water. The experiments were performed in a quiet room during the light phase between 8:00 A.M. and 14:00 P.M. The Ethics Committee of the Golestan University of Medical Sciences (Gorgan, Iran) approved the protocol of the current research. Enough efforts were always made to reduce the number of animals used in the current research and their suffering.
3.2. Scopolamine and Ginkgo biloba Extract Administration
The rats were randomly assigned to one of the six groups with six rats in each group including:
- Control group: not receiving the treatment.
- Sham-scopolamine group: Scopolamine injection (3 mg/kg, intraperitoneally (i.p.)) on the first day (18).
- Treatment groups (two groups): Scopolamine injection on the first day (3 mg/kg, i.p.), Ginkgo biloba extract administration (40 and 80 mg/kg/day, i.p.) (18) for seven days.
- Pretreatment groups (two groups): Ginkgo biloba extract administration (40 and 80 mg/kg/day, i.p.) for seven days, injection of scopolamine on the eighth day (3 mg/kg, i.p.).
Scopolamine hydrobromide (Tocris, UK) and Ginkgo biloba extract (NIAC Pharmaceutical Factory, Iran) were dissolved in sterile saline (0.9% NaCl).
3.3. Tissue Collection
At the end of the experimental sessions, the rats were deeply anesthetized with chloroform. Then, the brain tissues were removed and fixed in 4% paraformaldehyde for two weeks. Histological processing was done with the automated tissue processor machine (Did Sabz, Urmia, Iran). Next, 6-µm-thick sagittal sections were cut on a rotary microtome (Pooyan MK 1110, Mashhad, Iran) from the hippocampal formation (lateral 1.40 mm to 3.90 mm). 24 μm was the distance between every two consecutive sections. Then, the brain sections were stained with Congo red for Aβ plaques and Cresyl violet for neurons.
3.4. Congo Red Staining
Slides were stained with Congo red according to the manufacturer’s protocol (Tek-Path kit, Turkey) for Aβ plaques. Briefly, the slides were deparaffinized in xylene and hydrated through graded ethanol. Next, the slides were incubated with 1% Congo red at 56ºC for 45 minutes. After being washed in lithium carbonate for 15 seconds, the slides were stained with Mayer’s hematoxylin for one minute. Finally, the slides were dehydrated with 96% and 100% ethanol, cleared in xylene, and coverslipped with Entellan (Merck, Germany).
3.5. Cresyl Violet Staining
The slides after deparaffinization by xylene and hydration by graded ethanol were stained with 0.02% Cresyl violet (Sigma, USA) and rinsed quickly by distilled water. Finally, the slides were dehydrated in graded ethanol, placed in xylene, and coverslipped with Entellan (19).
3.6. Cell Counting
Images from sections were taken by a light microscope (BX51, Olympus, Japan) attached to a digital camera (DP 72, Olympus, Japan) at 40 × magnification for the Cornu Ammonis 1 (CA1) and Cornu Ammonis 3 (CA3) areas and cingulate cortex and at 100 × magnification for the Dentate Gyrus (DG) area. Fields of 30000 µm2 for the CA1 and CA3 regions and cingulate cortex and 4800 µm2 for the DG area were selected randomly in each picture. The area density of congophilic amyloid plaques and neurons was measured using ImageJ software and they were counted manually (20). To perform an unbiased measurement, imaging and counting were performed blind to treatment.
3.7. Statistical Analysis
SPSS version 16.0 software (Armonk, NY, USA) was used for the statistical analysis. Data were presented as Mean ± SD. After the normality assessment was done (Shapiro-Wilk test), multiple comparisons between the group mean values were done by one-way analysis of variance (ANOVA) followed by LSD (least significant difference) post hoc test. P < 0.05 was supposed to be statistically significant.
4. Results
4.1. Ginkgo biloba Extract Decreased Scopolamine-Induced Congophilic Amyloid Plaque Accumulation
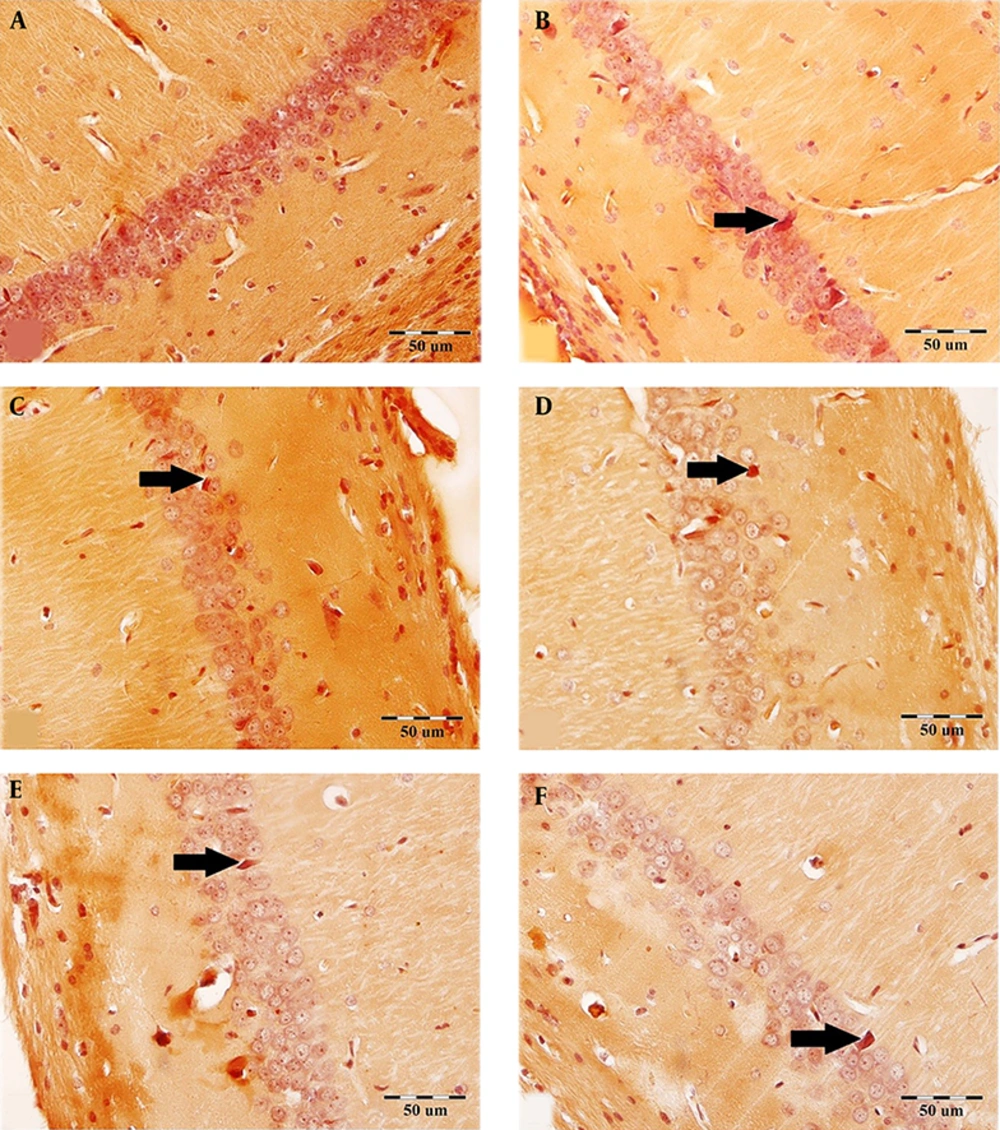
Figure 1 shows the effect of Ginkgo biloba extract on the scopolamine-induced congophilic amyloid plaque accumulation in the CA1 region.
The effect of Ginkgo biloba extract on the scopolamine-induced congophilic amyloid plaque accumulation in the CA1 region. The brain sections were stained with Congo red for amyloid β plaque. Arrows demarcate the amyloid β plaque in the CA1 region in all groups. A: Control, B: Sham-scopolamine, C: Treatment group, treated with scopolamine and 40 mg/kg/day Ginkgo biloba extract, D: Treatment group, treated with scopolamine and 80 mg/kg/day Ginkgo biloba extract, E: Pretreatment group, treated with 40 mg/kg/day Ginkgo biloba extract and scopolamine, F: Pretreatment group, treated with 80 mg/kg/day Ginkgo biloba extract and scopolamine.
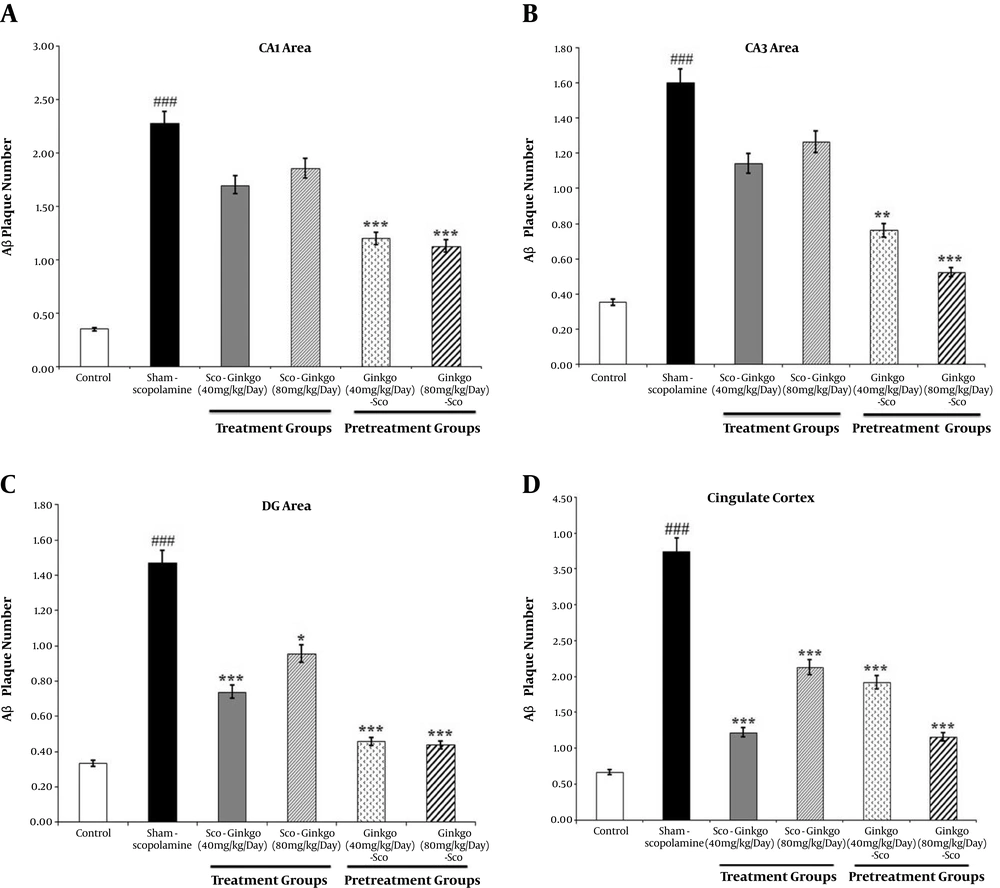
The administration of scopolamine remarkably increased the number of hippocampal congophilic amyloid plaques as compared to the control group. This increase in the CA1, CA3, and DG areas of the hippocampus (in the sham-scopolamine group) was statistically significant (P < 0.001, Figures 2A - C).
The effect of Ginkgo biloba extract on the scopolamine-induced congophilic amyloid plaque accumulation in the hippocampus (A, B, C) and cingulate cortex (D). Values represent mean. (###) P < 0.001 compared to the control group, (*) P < 0.05, (**) P < 0.01, and (***) P < 0.001 compared to the sham-scopolamine group.
Treatment with Ginkgo biloba extract decreased the number of congophilic amyloid plaques in all areas of the hippocampal formation compared to the sham-scopolamine group (Figures 2A - C). It seems in the DG area of the hippocampus, treatment with dose 40 mg/kg/day of the Ginkgo biloba extract effectively decreased the congophilic amyloid plaques density (0.74 ± 0.752, P ≤ 0.001, Figure 2C). Seven days pretreatment with the Ginkgo biloba extract considerably decreased the number of hippocampal congophilic amyloid plaques. The number of congophilic amyloid plaques in the hippocampal CA1, CA3, and DG areas significantly decreased by pretreatment with doses of 40 and 80 mg/kg/day of Ginkgo biloba extract (Figures 2A - C). The maximum decrease in the mean number of congophilic amyloid plaques in the hippocampal DG area was 0.46 ± 0.509 and 0.44 ± 0.512 at pretreatment with doses of 40 and 80 mg/kg/day of Ginkgo biloba extract, respectively.
The number of congophilic amyloid plaques increased in the cingulate cortex of the sham-scopolamine group while it significantly decreased following treatment and pretreatment with different doses of Ginkgo biloba extract (P < 0.001, Figure 2D).
4.2. Ginkgo biloba Extract Increased the Scopolamine-Induced Neuronal Loss
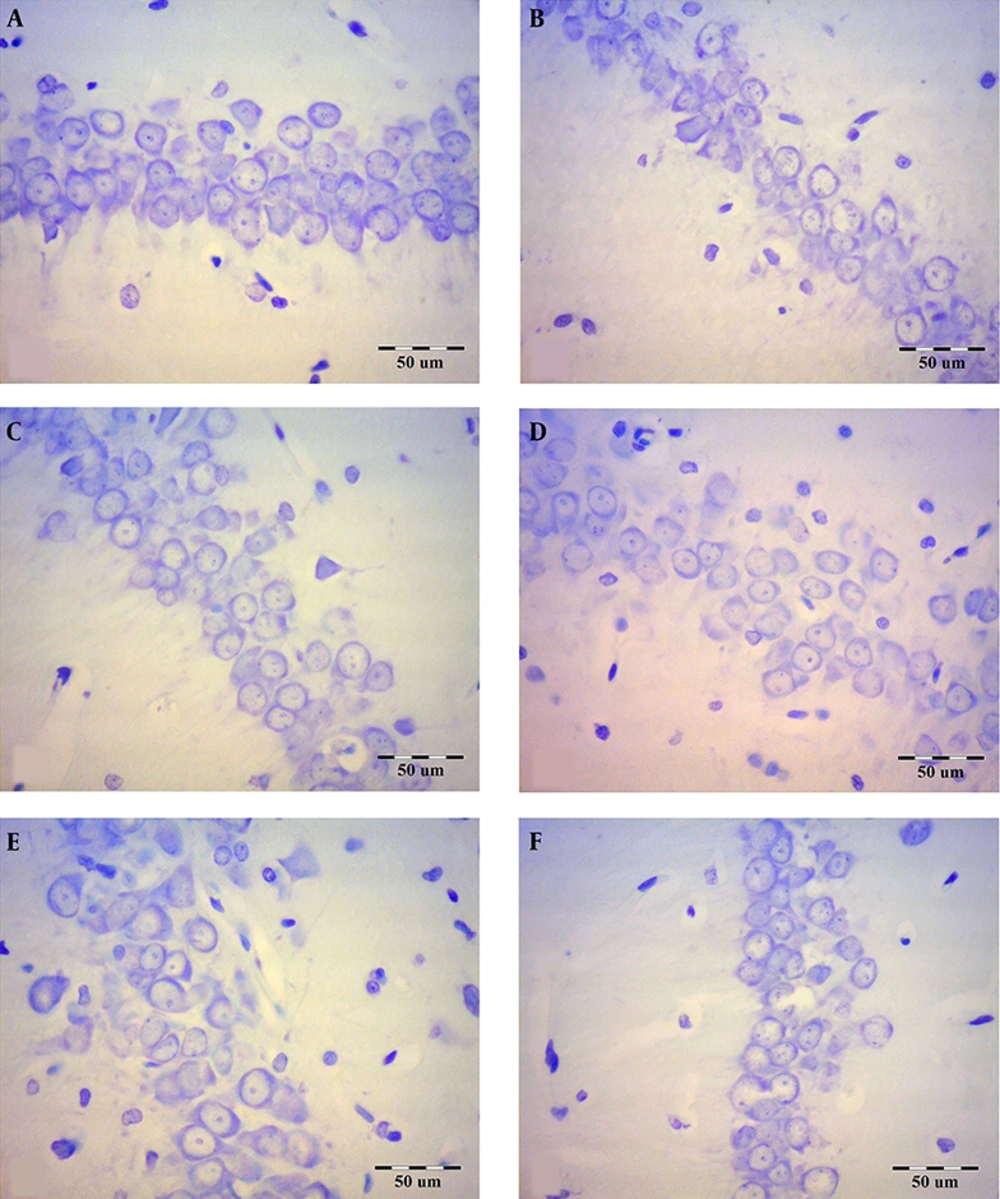
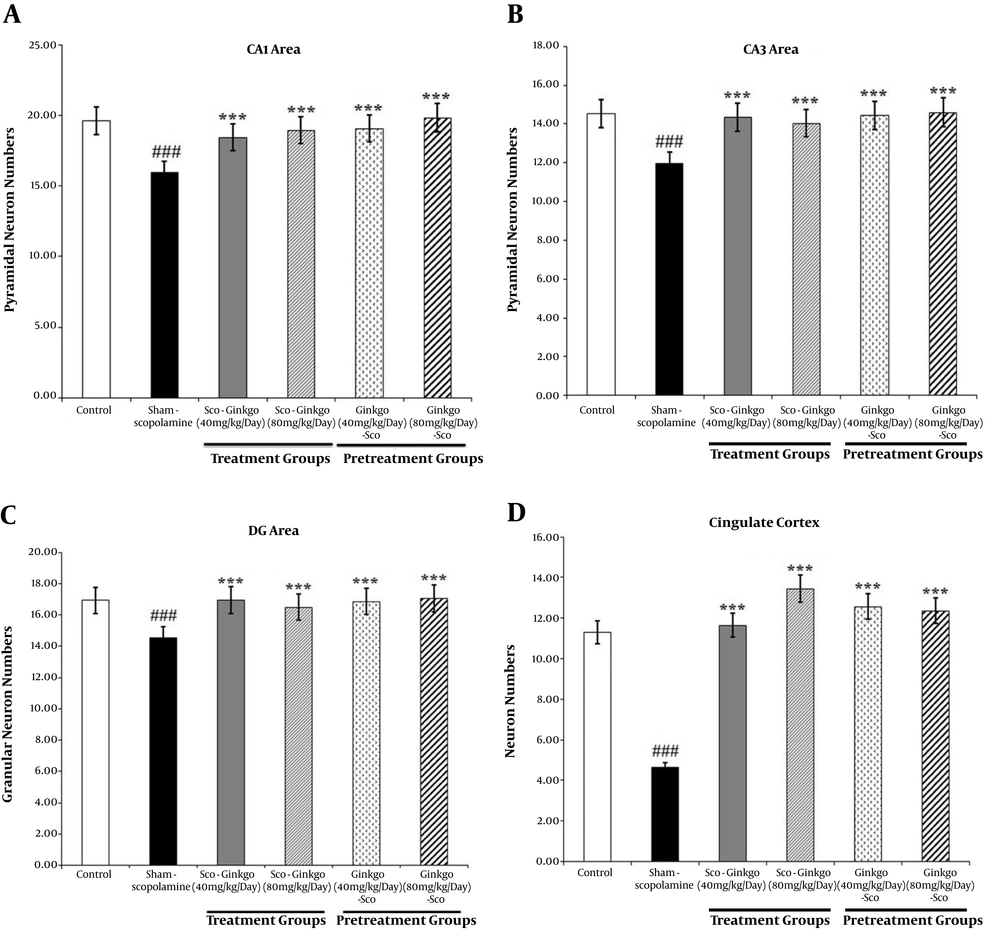
Figure 3 shows the effect of Ginkgo biloba extract on the number of hippocampal CA1 pyramidal neurons in scopolamine-treated rats.
The effect of Ginkgo biloba extract on the hippocampal CA1 pyramidal neuron numbers in scopolamine-treated rats. The brain sections were stained with Cresyl violet for neurons. A: Control, B: Sham-scopolamine, C: Treatment group, treated with scopolamine and 40 mg/kg/day Ginkgo biloba extract, D: Treatment group, treated with scopolamine and 80 mg/kg/day Ginkgo biloba extract, E: Pretreatment group, treated with 40 mg/kg/day Ginkgo biloba extract and scopolamine, F: Pretreatment group, treated with 80 mg/kg/day Ginkgo biloba extract and scopolamine.
Scopolamine reduced the number of pyramidal and granular neurons in all areas of the hippocampus significantly in comparison with the control group (P < 0.001; Figures 4A - C). The highest decrease in the mean number of pyramidal neurons in the hippocampal CA3 area was 11.95 ± 1.669 for the sham-scopolamine group. However, Ginkgo biloba extract could compensate for the reduction of the number of the hippocampal pyramidal and granular neurons before or after scopolamine administration. The maximum increase in the mean neuron numbers was 19.82 ± 2.736 in the hippocampal CA1 area at pretreatment with 80 mg/kg/day dose of Ginkgo biloba extract. Nevertheless, there was no significant difference between the protective and therapeutic groups of Ginkgo biloba extract.
Scopolamine significantly reduced the neuron numbers in the cingulate cortex in comparison with the control group (P < 0.001; Figure 4D). Treatment and pretreatment with Ginkgo biloba extract could significantly increase the neuron numbers in the cingulate cortex (P < 0.001; Figure 4D). The highest increase in the mean neuron numbers was observed in the treatment group with 80 mg/kg/day dose of Ginkgo biloba extract.
5. Discussion
The present study suggested that Ginkgo biloba extract could reduce scopolamine-induced congophilic amyloid plaques accumulation in the hippocampus and cingulate cortex. In addition, it was able to compensate for the reduction in hippocampal and cingulate cortex neuron numbers induced by scopolamine.
In this study, we found that a single dose of scopolamine could reduce the hippocampal pyramidal and DG granular neuron numbers. It is reported that scopolamine-induced memory deficit is probably associated with neuronal loss in a dose-dependent manner in the rat hippocampus (19). Some other research confirms scopolamine injection leads to severe cell losses in hippocampal cholinergic neurons (21, 22). Our findings on neuronal loss in scopolamine-treated rats are in agreement with these results.
Our study showed that both protective and therapeutic treatment with Ginkgo biloba extract could increase the hippocampal pyramidal and granular neuron numbers in scopolamine-treated rats. The effects of Ginkgo biloba extract on neuroprotection have been demonstrated in various studies. For example, a long-term administration of Ginkgo biloba extract in the aged Wistar rats enhanced dendritic arbors in the hippocampus CA pyramidal neurons (23). In addition, chronic treatment with Ginkgo biloba extract could prevent hippocampal CA1 pyramidal neuron damage in a model of transient global ischemia in gerbils (24). Pretreatment and treatment with Ginkgo biloba extract could attenuate scopolamine-induced apoptosis in rat hippocampal neurons (18). Similarly, in the present study, the sub-chronic administration of Ginkgo biloba extract in scopolamine-treated rats had positive effects on hippocampal neuron numbers.
Consistent with earlier findings (10, 25), our results showed that treatment of Wistar rats with scopolamine led to increased congophilic amyloid plaque accumulation in the hippocampus and cingulate cortex of the rats. Indeed, scopolamine-induced inhibition of muscarinic receptors led to an elevation in the Aβ level (25).
Interestingly, Ginkgo biloba extract also attenuated the scopolamine-mediated increase in congophilic amyloid plaque accumulation. In vitro data have suggested that the Ginkgo biloba extract inhibits Aβ aggregation (26). Furthermore, some reports indicate that Ginkgo biloba extract prevents Aβ-induced neurotoxicity with the inhibition of Aβ-induced events, for instance, reactive oxygen species production, glucose uptake, mitochondrial impairment, and apoptosis (27-29), where apoptosis is considered one of the main causes of neurodegenerative diseases and thus, helps relieve the AD (30).
Liu et al. (5) reported that long-term oral treatment (for five months) with Ginkgo biloba extract might reduce cerebral Aβ pathology by inhibiting β-secretase activity and Aβ aggregation. Whereas in this study, we found that Ginkgo biloba extract could reverse the increase of congophilic amyloid plaque numbers in the hippocampus and cingulate cortex within seven days before and after the injection of scopolamine. This finding indicates that the anti-AD effects of Ginkgo biloba extract require a sufficient duration of treatment (5).
Although, in this study, we did not investigate the mechanisms underlying the beneficial effects of Ginkgo biloba extract on the reduction of congophilic amyloid plaque density in scopolamine-treated rats, Liu et al. (5) demonstrated that Ginkgo biloba extract ameliorates AD pathogenesis by enhancing autophagy and inhibiting neuroinflammation. In addition, with other mechanisms, e.g., inhibiting Aβ aggregation and promoting neuroprotection and neurogenesis, Ginkgo biloba extract reduces synaptic loss and cognitive impairment in the AD (11).
5.1. Conclusion
Our results showed that Ginkgo biloba extract could play protective roles against some scopolamine-induced AD-like pathologic dysfunctions, including Aβ accumulation and neuronal loss, suggesting that treatment with Ginkgo biloba extract might be a promising prophylactic target for the AD.