Abstract
Background:
Bambusa vulgaris (bamboo) is a common plant in tropical regions of the world, with multiple biological activities. Cereal steep liquors have been identified to act against some fecal bacteria. Given the increasing prevalence of antimicrobial resistance coupled with the cost of antibiotics, there is a need for mitigation efforts and the shift by locals to herbal products.Methods:
The factorial study design was employed. Bamboo leaves were washed, dried, milled, and soxhlet extracted for 16 h using the steep fermented liquor from Zea mays subsp. mays as the solvent. Rotary evaporated extracts of various weights were used to carry out sensitivity and minimum inhibitory concentration (MIC) tests. Oral acute toxicity was determined. The in vivo assay was used to determine the effective dosage for the treatment of infected mice while distilled water and ciprofloxacin served as negative and positive controls, respectively.Results:
The average inhibition zone diameters in the sensitivity test at 25, 50, and 100 mg/mL concentrations were 16.20 ± 0.06 mm, 19.10 ± 0.02 mm, and 22.10 ± 0.05 mm, respectively, while the MIC against Salmonella typhi was 25 mg/mL. The extract was found to be safe at up to 5000 mg/kg dose, which means that the LD50 was ≥ 3808 mg/kg. The Widal test revealed that mice responded to treatment with the extract at different concentrations (50, 100, and 200 mg/mL) as the number of days increased. The in vivo assay on day 14 showed the bactericidal property at a dosage of 200 mg/mL.Conclusions:
Bamboo leaves extract in steep liquor from fermented yellow maize displayed excellent pharmacological activity against the pathogenic organism of typhoid fever with a potential source of active agents.Keywords
Salmonella typhi Toxicology Ethnomedicine Bambusa vulgaris Fermentation Zea mays
1. Background
The deadly threat to human health by infectious diseases such as typhoid fever and other relevant disorders is felt by a vast majority of tropical countries due to limited chemotherapy and nonavailability of vaccines (1). Given the increasing prevalence of antimicrobial resistance coupled with the cost of antibiotics and its associated clinical implications, there is a need for mitigation efforts. Nowadays, people are switching to natural products over synthetic compounds, which can be easily obtained from locally available plants and can help in reducing public health costs. The use of natural products is playing a pivotal role in developing countries' healthcare delivery system due to the earning power or poverty level of the people (2). In Africa, many plant species are used traditionally to cure infectious diseases; hence, fighting infections using natural products will be advantageous and affordable to most local patients (3) in the face of the increasing cost of orthodox medicines.
Salmonella infection is rated among the most common food-borne diseases worldwide. The persisting morbidity and mortality of Salmonella infections, primarily S. typhi infection, may be attributed to the increasing resistance rate of this pathogen to a few commonly used antimicrobial compounds (4) in many developing countries. Thus, novel, efficient, safe, and affordable remedies for typhoid fever or salmonellosis are necessary for these countries.
Bambusa vulgaris (bamboo) leaves contain higher protein levels than the stem. The aqueous extract of matured bamboo leaves contains six phenolic acids, including chlorogenic acid, ferulic acid, coumaric acid, protocatechuic acid, vanillic acid, and caffeic acid. These phenolic acids might be responsible for the allelopathic (weedicidal) effects (5, 6). Bamboo leaves are used to treat febrile disease, thirst-related symptoms, and palsy, and they are an effective treatment of cough, phlegm, and fever. They are also used for treating hemoptysis and swelling, inducing urination, and stopping bleeding (7).
The most commonly used diagnostic test for typhoid fever in developing countries like Nigeria is the Widal test. The levels of the agglutinating antibody against O and H antigens are used for Salmonella typhi. The serological response in acute typhoid fever raises the titer value of the O antibody, with an elevation of the H antibody titer developing more slowly (8).
Earlier studies have proven that steep fermented liquor from different grains has appreciable antimicrobial activity against test organisms (9, 10). This finding was irrespective of the steeping procedure used. This study aimed to determine the dosage and toxicity level in the ethnopharmacological use of fermented maize liquor extract of Bambusa vulgaris leaves for the treatment of typhoid fever in Nigeria.
2. Objectives
The study provides scientific evaluation for treatment claims and accurately disseminates better information to people regarding efficient treatment and improved health status. The extraction of bamboo leaves with fermented maize steep liquor alines with the 1991 WHO guidelines for the assessment of herbal medicine and the sixth International Conference of Drug Regulatory Authorities held in Ottawa for quality assessment, stability, safety assessment, and efficacy assessment (11).
3. Methods
3.1. Preparation of Fermented Maize Steep Liquor
The steep liquor from Zea mays subsp. mays (yellow maize) was produced from clean and healthy grains bought from the market in Akungba community in Ondo-State, Nigeria. Then, 1.5 kg of the grains were washed thoroughly, submerged in 3.5 liters of clean tap water, and allowed to ferment for four days. The fermented maize was milled and sieved with a sterile cotton sieve. The filtrate was fermented for 24 h, and the steep maize liquor, which was a fluid free from sediments, was stored in plastic kegs in a refrigerator at 4°C for further use (9).
3.2. Crude Extraction of Bamboo Leaves Components with Fermented Maize Steep Liquor
Bambusa vulgaris Schrad. Ex. J.C. Wendel (Bamboo) leaves, the Poaceae family, was collected at the Faculty of Pharmacy Building, Obafemi Awolowo University, Ile-Ife, Nigeria. The voucher specimen deposited at the Faculty of Pharmacy Herbarium included in the index herbarium online, with voucher number FPI 2204. Leaves were oven-dried at 60°C and ground using a mechanical grinder to obtain a smooth powder. One kilogram of the powdered bamboo leaves was extracted with seven liters of fermented corn steep liquor using the hot Soxhlet extraction method for 8 h (12). The extract was filtered, concentrated with a rotary evaporator (Stroglass, Italy) at 50°C under reduced pressure, and lyophilized with a freeze drier (Zirbus, Germany) (13). The percentage yield was 12.24%.
3.3. Microorganism Suspension Standardization
Salmonella typhi (ST0042) used for this experiment was obtained from the Nigerian Institute of Medical Research Laboratory (NIMRL), Yaba, Lagos. The activation of the isolates was carried out by culturing on Salmonella-Shigella agar (Difco) and incubated at 37°C for 24 h. The pure culture subcultured on Salmonella-Shigella broth (Difco) at 37°C for 18 h.
The standardized culture of Salmonella typhi (ST0042) to the 0.5 McFarland concentration
was used with approximately 1 to 2 × 108 CFU/mL concentrations of young cultures (14).
3.4. Antimicrobial Property
3.4.1. Screening of the Extract for Antibacterial Activity
Fifteen portions of 18.0 mL of Muller Hinton agar (Scharlua, Spain) were prepared, sterilized, and left to cool to about 47°C. One milliliter of the 18 h Salmonella typhi (ST0042) broth culture was seeded into each of the agars. The seeded agars were poured into sterile Petridishes and labeled accordingly (15). Then, 100.0 mg/mL, 50.0 mg/mL, 25.0 mg/mL concentrations of the crude extract were weighed in triplicate into distilled water for reconstitution, while ciprofloxacin and distilled water served positive and negative controls, respectively. A sterile cork borer of 8 mm diameter was used to make three wells in each of the seeded agars and 0.1 mL of each concentration of the crude extract was dispensed into the wells with a sterile micropipette. The plates were left for 30 min for the extract to diffuse into the agar and later kept in an incubator for 24 h at 37°C. The inhibition zone diameter was measured and recorded. The experiments were performed in triplicate.
3.4.2. Determination of the Minimum Inhibitory Concentration
Crude extracts at concentrations 50, 25, 12.5, 6.25, 3.125, and 1.56 mg/mL were prepared in sterilized McCartney bottles. Then, 18 mL of sterilized molten Muller Hinton agar (Scharlua, Spain) was added to each extract diluent at 45°C, mixed well by rolling between palms, poured aseptically into sterile Petri dishes, and allowed to gel. A loop full of 18 h Salmonella-Shigella broth culture was streaked on respective plates containing different concentrations of the extract and incubated at 37°C for 24 h. The concentration at which there was no visible growth indicated the minimum inhibitory concentration (14). Ciprofloxacin was tested at the same concentrations.
3.5. Determination of the Acute Toxicity of the Extract
The OECD method (16) was used for acute toxicity measurement, which involved two phases. Twenty-two mice were weighed and divided into two groups for the first and second phases of the test. Twelve mice were used for the first phase and the other ten mice for the second phase. The control group was administered normal saline while the others were administered a dosage of the extract. The determination of the acute toxicity started with using 10 mg/kg, 100 mg/kg, and 1000 mg/kg bodyweight for the first phase and then increased to 1000 mg/kg, 1600 mg/kg, 2900 mg/kg, and 5000 mg/kg body weight in the second phase, the results of which were used to determine the LD50. The different body weight of the mice determined the dosage of the extract and were marked for easy identification. The mice used were denied food for almost five hours before the test. The bamboo extract was administered to them orally with the aid of a canular, and mice were closely monitored for 24 h.
3.6. Dosage Determination
3.6.1. Dosage Determination of Extract
A total of 25 Albino mice (male and female) were used and randomly distributed into five test groups of five mice each. The working doses should be less than 50% of the LD50, which was > 5000 mg/kg (17). The dosages were 250 mg/kg body weight (group B), 500 mg/kg (group C), and 1000 mg/kg (group D). Distilled water was administered to group A as the negative control group, while 500 mg/kg bodyweight ciprofloxacin, a commercial antibiotic, was used as the positive control given to group E. A 10 mL of 18 h Salmonella-Shigella broth (Difco) culture of Salmonella typhi was prepared and 1.0 mL of the culture was given orally to the test groups A-E on day zero. The calculated doses in each group per average weight per mouse were given from day zero (18).
3.6.2. In vivo Assay of Salmonella Infection
The Widal agglutination test was carried out using HiPer® 169 Widal test Teaching Kit (Slide Test; code: HTI017; HiMedia Laboratories Pvt. Limited, Mumbai-400 086, India) to determine the extent of Salmonella infection in experimental animals. The consecutive treatments of both extracts and controls on days one, seven, and 14 were employed for the agglutination test. The test sera were obtained by collecting blood from the tail of mice at dilutions of 80 µL, 40 µL, 20 µL, 10 µL, and 5 µL each for four antigens. The experiments followed the manufacturer’s specification and quantified using the procedure for the serial titer value of Salmonella antibodies. The level of infection was estimated depending on the extent of the clump’s formation of blood samples according to the modified technique of Akinyemi et al. (19).
3.7. Gross Behavioural Studies of Mice for Dosage Determination
The gross behavioral changes in albino mice due to the administration of different doses were observed at scheduled time intervals (6).
3.8. Statistical Analysis
The one-way Analysis of Variance (ANOVA) was used for determining the statistical differences of various extract concentrations between the control and test groups. The levels of significance were set at 0.05 and 0.01 (20). SPSS for Windows version 17 was used for statistical analysis.
4. Results
The percentage yield of the crude extract of bamboo leaves with steep corn liquor was 12.24%. In the Salmonella typhi sensitivity test, there was an increase in the diameter of the clear zone from 16.20 ± 0.66 to 22.10 ± 0.05 mm as the concentration of B. vulgaris steep liquor extract increased from 25.00 to 100.00 mg/mL (Table 1). The level of sensitivity with ciprofloxacin (21.30 ± 0.04 to 38.40 ± 0.05) increased significantly at the same concentration. The result of the MIC assay (Table 2) revealed complete clarity, indicating no observable growth at levels of 25 mg/ml and 50 mg/mL of the crude extract. Hence, the minimum inhibitory concentration was 25 mg/mL. The ciprofloxacin MIC assay showed clarity, with no observable growth at 3.125 mg/mL.
The Activity of Yellow Maize Fermented Liquor Extract of Bambusa tuldoides cv. ventricosa Against Salmonella typhia, b
| Concentration, mg/mL | Zone of Inhibition, mm |
|---|---|
| Sensitivity activity of crude extract | |
| 25.00 | 16.20 ± 0.06 A |
| 50.00 | 19.10 ± 0.02 B |
| 100.00 | 22.10 ± 0.05 C,D |
| Sensitivity activity of ciprofloxacin | |
| 25.00 | 21.30 ± 0.04 C |
| 50.00 | 28.10 ± 0.02 E |
| 100.00 | 38.40 ± 0.05 F |
Minimum Inhibitory Concentration (MIC) of Yellow Maize Fermented Liquor Extract of Bambusa tuldoides cv. ventricosa Against Salmonella typhia
| Concentration, mg/mL | Observation |
|---|---|
| Crude extract | |
| 50 | No visible growth |
| 25 | No visible growth |
| 12.5 | Visible growth |
| 6.25 | Visible growth |
| 3.125 | Visible growth |
| 1.56 | Visible growth |
| Ciprofloxacin | |
| 50 | No visible growth |
| 2 | No visible growth |
| 12.5 | No visible growth |
| 6.25 | No visible growth |
| 3.125 | No visible growth |
| 1.56 | Visible growth |
The phase I acute toxicity showed no death in all the groups. Only one animal died in the second phase at the highest concentration administered (5000 mg/kg body weight). Hence, the acute toxicity of the crude extract of bamboo leaves was less than 3808 mg/kg body weight. [The formula for LD50 was √ (D0 × D100) where D0 is the highest dose with no mortality, and D100 is the highest dose with mortality; in this case: √ (2900 × 5000)].
Tables 3-5 show the result of the Widal test assay for the confirmation of S. typhi infection in experimental mice. Agglutination in each of these assays was noted. 80 µL corresponded to 1 in 20 dilutions, 40 µL to 1 in 40, 20 µL to 1 in 80, 10 µL to 1 in 160, and 5 µL corresponded to 1 in 320 titers. On day 1, the effectiveness of all the three dosages (O antigen 1/160; H antigen-1/160) was not significantly different for S. typhi from the effectiveness of ciprofloxacin as the positive control (O antigen-1/160; H antigen-1/160) (Table 3). On day 7 of continuous administration of the extract, there was a significant reduction in O and H antigens with the increased dose (Table 4). The bamboo leaf extract given at a treatment dosage of 50 mg/mL had the highest O antigen of 1/80, with its H antigen of 1/80 for S. typhi, compared to the treatment dose of 200 mg/mL with a lower O antigen of 1/40 and H antigen of 1/40.
Widal Test for the Presence of Salmonella typhi on Day One in Mice Groups Treated with Different Extract and Control Dosages
| Microbial Strain | O Antigen | H Antigen |
|---|---|---|
| Group A 10 mg/mL distilled water | ||
| Salmonella typhi | 1/160 | 1/160 |
| Salmonella paratyphi A | 1/80 | 1/80 |
| Salmonella paratyphi B | 1/80 | 1/80 |
| Salmonella paratyphi C | 1/160 | 1/80 |
| Group B 50 mg/mL extract | ||
| Salmonella typhi | 1/160 | 1/160 |
| Salmonella paratyphi A | 1/80 | 1/80 |
| Salmonella paratyphi B | 1/80 | 1/80 |
| Salmonella paratyphi C | 1/160 | 1/80 |
| Group C 100 mg/mL extract | ||
| Salmonella typhi | 1/160 | 1/160 |
| Salmonella paratyphi A | 1/80 | 1/80 |
| Salmonella paratyphi B | 1/80 | 1/80 |
| Salmonella paratyphi C | 1/160 | 1/80 |
| Group D 200 mg/mL extract | ||
| Salmonella typhi | 1/160 | 1/160 |
| Salmonella paratyphi A | 1/80 | 1/80 |
| Salmonella paratyphi B | 1/80 | 1/80 |
| Salmonella paratyphi C | 1/160 | 1/80 |
| Group E 100 mg/mL ciprofloxacin | ||
| Salmonella typhi | 1/160 | 1/160 |
| Salmonella paratyphi A | 1/80 | 1/80 |
| Salmonella paratyphi B | 1/80 | 1/80 |
| Salmonella paratyphi C | 1/160 | 1/80 |
Widal Test for the Presence of Salmonella typhi on Day Seven in Mice Groups Treated with Different Extract and Control Dosages
| Microbial Strain | O Antigen | H Antigen |
|---|---|---|
| Group A 10 mg/mL distilled water | ||
| Salmonella typhi | 1/160 | 1/160 |
| Salmonella paratyphi A | 1/80 | 1/160 |
| Salmonella paratyphi B | 1/80 | 1/160 |
| Salmonella paratyphi C | 1/160 | 1/160 |
| Group B 50 mg/mL extract | ||
| Salmonella typhi | 1/80 | 1/80 |
| Salmonella paratyphi A | 1/80 | 1/80 |
| Salmonella paratyphi B | 1/80 | 1/80 |
| Salmonella paratyphi C | 1/80 | 1/80 |
| Group C 100 mg/mL extract | ||
| Salmonella typhi | 1/40 | 1/80 |
| Salmonella paratyphi A | 1/40 | 1/80 |
| Salmonella paratyphi B | 1/40 | 1/80 |
| Salmonella paratyphi C | 1/40 | 1/80 |
| Group D 200 mg/mL extract | ||
| Salmonella typhi | 1/40 | 1/40 |
| Salmonella paratyphi A | 1/40 | 1/40 |
| Salmonella paratyphi B | 1/40 | 1/20 |
| Salmonella paratyphi C | 1/20 | 1/40 |
| Group E 100 mg/mL ciprofloxacin | ||
| Salmonella typhi | 1/20 | 1/40 |
| Salmonella paratyphi A | 1/40 | 1/20 |
| Salmonella paratyphi B | 1/20 | 1/20 |
| Salmonella paratyphi C | 1/40 | 1/40 |
Widal Test for the Presence of Salmonella typhi on Day 14 in Mice Groups Treated with Different Extract and Control Dosages
| Microbial Strain | O Antigen | H Antigen |
|---|---|---|
| Group A 10 mg/mL distilled water | ||
| Salmonella typhi | 1/160 | 1/160 |
| Salmonella paratyphi A | 1/160 | 1/80 |
| Salmonella paratyphi B | 1/80 | 1/80 |
| Salmonella paratyphi C | 1/160 | 1/80 |
| Group B 50 mg/mL extract | ||
| Salmonella typhi | 1/40 | 1/80 |
| Salmonella paratyphi A | 1/40 | 1/80 |
| Salmonella paratyphi B | 1/40 | 1/80 |
| Salmonella paratyphi C | 1/40 | 1/80 |
| Group C 100 mg/mL extract | ||
| Salmonella typhi | 1/40 | 1/40 |
| Salmonella paratyphi A | 1/40 | 1/40 |
| Salmonella paratyphi B | 1/40 | 1/40 |
| Salmonella paratyphi C | 1/40 | 1/40 |
| Group D 200 mg/mL extract | ||
| Salmonella typhi | 1/20 | 1/20 |
| Salmonella paratyphi A | 1/40 | 1/20 |
| Salmonella paratyphi B | 1/20 | 1/20 |
| Salmonella paratyphi C | 1/20 | 1/20 |
| Group E 100 mg/mL ciprofloxacin | ||
| Salmonella typhi | 1/20 | 1/20 |
| Salmonella paratyphi A | 1/20 | 1/20 |
| Salmonella paratyphi B | 1/20 | 1/20 |
| Salmonella paratyphi C | 1/20 | 1/20 |
Mice treated with 500 mg/kg of the crude extract (100 mg/mL) showed no significant difference between their O and H antigens (1/40 and 1/80, respectively) for S. typhi.
The group given the dosage of 1000 mg/kg of crude extract (200 mg/mL) showed a high organism reduction in the body of infected animals (O antigen-1/40; H antigen-1/40). The mice group treated with ciprofloxacin showed a highly significant reduction compared to the crude extract for the S. typhi antigen (O-1/20; H-1/40), while that of the negative control group had O and H antigens of 1/160 (Table 4). The Salmonella typhi antigen in treated groups on day 14 decreased significantly with increasing dosage. The group treated with the highest dosage of 1000 mg/kg of crude extract of bamboo leaves had the highest reduction in both O and H antigen of S. typhi (1/20); this was in comparison with the O and H antigens of S. typhi of mice treated with ciprofloxacin (1/20). The antigens of S. typhi the negative control group increased from day 1 to 7 but there was no significant increase from day 7 to 14 (1/160) (Table 5). Agglutination assays used to determine the concentrations of specific antibodies in mice immune sera revealed that the anti-Salmonella antibodies in infected mice gradually reduced in a dose-dependent manner.
4.1. Observable Symptoms
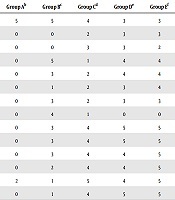
At different phases of the acute toxicity test, no signs of dullness were observed in all mice (Table 6). Only mice administered with 5000 mg/kg body weight of the extract showed little signs of paleness.
Gross Behavioural Studies of Mice Infected with Salmonella typhi and Treated with Yellow Maize/Bamboo Leaf Extract from Day 1 to Day 14a
| Days of Treatment | Mice Behavioural Characteristics | Group Ab | Group Bc | Group Cd | Group De | Group Ef |
|---|---|---|---|---|---|---|
| 1 | Alertness | 5 | 5 | 4 | 3 | 3 |
| 2 | Loss of appetite | 0 | 0 | 2 | 3 | 3 |
| 3 | Loss of weight | 0 | 0 | 3 | 3 | 2 |
| 4 | Reduced activity | 0 | 5 | 1 | 4 | 4 |
| 5 | Swollen libs | 0 | 3 | 2 | 4 | 4 |
| 6 | Constriction of pupils | 0 | 1 | 2 | 3 | 4 |
| 7 | Drowsiness | 0 | 3 | 2 | 3 | 3 |
| 8 | Alertness | 0 | 4 | 1 | 0 | 0 |
| 9 | Loss of appetite | 0 | 3 | 4 | 5 | 5 |
| 10 | Loss of weight | 0 | 3 | 4 | 5 | 5 |
| 11 | Reduced activity | 0 | 3 | 4 | 4 | 5 |
| 12 | Swollen libs | 0 | 2 | 4 | 4 | 5 |
| 13 | Constriction of pupils | 2 | 1 | 5 | 4 | 5 |
| 14 | Drowsiness | 0 | 1 | 4 | 5 | 5 |
All mice treated with distilled water showed the persistence of catatonic, ataxia, lethargy, respiratory distress, dehydration, and weight loss as their common behavioral characteristics. Group B mice, treated with 50 mg/mL of the extract, initially had a similar persistence of catatonic, ataxia, lethargy, respiratory distress, dehydration, and weight loss but manifested a brief improvement in these behavioral characteristics as the treatment approached the 14th day. Group C mice treated with 100 mg/mL of the extract showed drastic improvements in their behavioral characteristics from the ninth day of treatment to the 14th day of treatment. Group D treated with 200 mg/mL of the extract and group E treated with 100 mg/mL of ciprofloxacin manifested similar sharp improvements in their behavioral characteristics in terms of catatonic, ataxia, lethargy, respiratory distress, dehydration, and weight loss. Group D started to manifest improved characteristics from the seventh day of treatment, while the behavioral improvement in the control group E started to improve from the fourth day of treatment.
5. Discussion
In this study, the sensitivity test values showed that the corn liquor extract of bamboo leaf demonstrated an appreciably high activity on Salmonella typhi, with an increase in the inhibition zone diameters of the organism as concentration increased. This revealed that the action of the extract was concentration-dependent. The MIC results also showed that at 25 mg/mL, the extract completely inhibited the growth of Salmonella. This is in line with the findings of Adebolu et al. (9). The inclusion of bamboo leaf extract may improve the activity of the fermented liquor by increasing its scope of action beyond common diarrheal bacteria. The biologically active components of bamboo leaves may be responsible for the above attributes. These components are saponins, phenols (anthraquinones and coumarins), tannins, and alkaloids, all of which are relatively strong antibacterial and bactericidal agents (21). Also, there is the capability of the fermented steep liquor to enhance the activities of these active components in bamboo leaves. The presence of lactobacilli in the cereal fermented steep liquor produced compounds such as organic acids, diacetyl alcohol, hydrogen peroxide, and bacteriocins, which have inhibitory activities against pathogenic organisms, including Salmonella typhi (9, 22) and regarded as a major factor explaining why the extraction of bamboo leaves biomolecules was useful in this study (21).
The probable modes of action of phenolic compounds as antimicrobial agents, which are significant components of bamboo leaves (5, 6, 21), are not explicit. The effect of phenolic compounds can be concentration-dependent. At low concentrations, phenols can affect enzyme activity, particularly those associated with energy production, while at high levels, they can cause protein denaturation. The antimicrobial effect of phenolic compounds has its ability to alter cell permeability, hence permitting the loss of macromolecules. They also interfere with membrane function (electron transport, nutrient uptake, protein synthesis, nucleic acid synthesis, and enzyme activity). Phenolic compounds interact with membrane proteins, causing deformation in structure and functionality. The high antibacterial activity of phenolic components can be explained by alkyl substitution into the phenol nucleus (23).
The viewpoint of concentration-dependent activity of the extract is acceptable so long as the toxicity level will be high enough to allow for an increased amount of consumption dosage. Aprevious investigation by Gabriel-Ajobiewe et al. (24), revealed that the fermented steep liquor extracts of Bambusat uldoides cv. ventricosa leaves were active against bacterial species. The result of acute toxicity showed the median lethal dose (LD50) of the extract was 3808 mg/kg body weight of experimental animals, indicating the safety of the leaves of the plant (25). The result of the Widal test also revealed the activity of the extract against Salmonella in vivo (Table 6). The effectiveness of the extract increased with days and concentrations consumed by experimental mice.
The findings of this study suggested that the extract was bacteriostatic at lower concentrations while displaying bactericidal activity at higher levels according to Tiwari et al. study (25).
The decrease in antigen titer as the concentration increased was revealed coupled with the length of days progressed. Serum agglutinin showed the level of agglutination, and the higher the level of agglutination, e.g., 1/160, the higher the presence of S. typhi antigen and vice versa. The fact that S. typhi possesses capsular polysaccharides (VI antigen) (26), which makes it more virulent than other serotypes implicated in typhoidal-salmonellosis, can result in the above action.
Moreover, the potent antimicrobial effect of bamboo leaves extracts with the steep fermented liquor on S. typhi infection may be due to the mixture of bioactive compounds present compared to the pure compound in standard antibiotics (6).
The manifestation of the persistent behavioral characteristics of catatonic, ataxia, lethargy, respiratory distress, dehydration, and weight loss in mouse model infection treated with placebo agrees with the findings of some researchers (27-29). The demonstration of the reversal of behavioral characteristics of the mice model at higher doses in comparison with ciprofloxacin antibiotic tallies with the findings of Talukdar et al. (27) and Panda et al. (28).
A detailed consideration of various disadvantages associated with the use of antibiotics and the rather small disparity between the effectiveness of bamboo leaf extract and the standard drug used make the exploitation of extracts from medicinal plants such as bamboo an attractive alternative in the early treatment of typhoid and paratyphoid fever, together with other diseases caused by non-typhoidal Salmonella.
5.1. Conclusions
The results obtained in this experiment showed that Zea mays subsp. mays fermented liquor extract of the bamboo leaf can be a potential source of active bio-agents and if subjected to further studies and processing procedures, could be employed as a medicinal drink in the treatment of common infectious diseases caused by Salmonella species. The introduction of pharmaceutical materials into the human food chain may be prospective for pharmaceutical products from plants for use in food crops. This is in line with the advocacy of the World Health Organization for countries through their interactions with traditional medicines to identify and exploit aspects that provide safe and effective remedies for ailments of both microbial and non-microbial origins (11).
References
-
1.
Crump JA, Mintz ED. Global trends in typhoid and paratyphoid Fever. Clin Infect Dis. 2010;50(2):241-6. [PubMed ID: 20014951]. [PubMed Central ID: PMC2798017]. https://doi.org/10.1086/649541.
-
2.
Gupta S, Parvez N, Bhandari A, Sharma P. Microspheres based on herbal actives: the less-explored ways of disease treatment. Egypt Pharm J. 2015;14(3):148. https://doi.org/10.4103/1687-4315.172852.
-
3.
Kuete V. Potential of Cameroonian plants and derived products against microbial infections: a review. Planta Med. 2010;76(14):1479-91. [PubMed ID: 20533165]. https://doi.org/10.1055/s-0030-1250027.
-
4.
Helms M, Ethelberg S, Molbak K, D. T. Study Group. International salmonella typhimurium DT104 infections, 1992-2001. Emerg Infect Dis. 2005;11(6):859-67. [PubMed ID: 15963280]. [PubMed Central ID: PMC3367607]. https://doi.org/10.3201/eid1106.041017.
-
5.
Zhang Y, Yao X, Bao B, Zhang Y. Anti-fatigue activity of a triterpenoid-rich extract from Chinese bamboo shavings (Caulis bamfusae in taeniam). Phytother Res. 2006;20(10):872-6. [PubMed ID: 16886233]. https://doi.org/10.1002/ptr.1965.
-
6.
Zhang Y, Tie X, Bao B, Wu X, Zhang Y. Metabolism of flavone C-glucosides and p-coumaric acid from antioxidant of bamboo leaves (AOB) in rats. Br J Nutr. 2007;97(3):484-94. [PubMed ID: 17313710]. https://doi.org/10.1017/S0007114507336830.
-
7.
Baker JH, Qiu J, Grine K. Role of complementary and alternative therapies in infectious disease. Prim Care. 2018;45(3):533-9. [PubMed ID: 30115339]. https://doi.org/10.1016/j.pop.2018.05.009.
-
8.
Aziz T, Haque SS. Role of widal test in the diagnosis of typhoid fever in context to other test. Am J Biochem. 2012;2(1):16-8. https://doi.org/10.5923/j.ajb.20120201.04.
-
9.
Adebolu TT, Olodun AO, Ihunweze BC. Evaluation of ogi liquor from different grains for antibacterial activities against some common diarrhoeal bacteria in Southwest Nigeria. Afri J Biotechn. 2007;6(9):1140-3. https://doi.org/10.5897/ajb2007.000-2151.
-
10.
M B, M O, A M. Comparative antimicrobial potentials of omidun obtained from yellow and white maize varieties on some diarrhoea causing microorganisms. Afri J Microbiol Res. 2016;10(27):1018-23. https://doi.org/10.5897/ajmr2015.7870.
-
11.
WHO. Expert committee on specifications for pharmaceutical preparations - Thirty-fourth Report. World Health Organization; 1996. Report No.: 863.
-
12.
Pandey AK. Anti-staphylococccal activity of a pan-tropical aggressive and obnoxious weed Parthenium histerophorus: an in vitro study. Natl Acad Sci Lett. 2007;30:383-6.
-
13.
Kaikini AA, Dhande SR, Kadam VJ. Overview of indian medicinal tree: Bambusa bambos (druce). Int Res J Pharm. 2013;4(8):52-6. https://doi.org/10.7897/2230-8407.04809.
-
14.
CLSI. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard. CLSI document M07-a10. Wayne, PA: Clinical and Laboratory Standards Institute; 2015. Available from: https://clsi.org/media/1632/m07a10_sample.pdf.
-
15.
Parekh J. In vitro screening of antibacterial activity of aqueous and alcoholic extracts of various Indian plant species against selected pathogens from Enterobacteriaceae. Afri J Microbiol Res. 2007;1(6):92-9.
-
16.
OECD. Acute oral toxicity - acute toxic class method 423 adopted, guideline for testing of chemicals. 2001. Available from: https://ntp.niehs.nih.gov/iccvam/suppdocs/feddocs/oecd/oecd_gl423.pdf.
-
17.
Anisuzzman M, Hasan MM, Acharzo AK, Das AK, Rahman S. In vivo and in vitro evaluation of pharmacological potentials of secondary bioactive metabolites of dalbergia candenatensis leaves. Evid Based Complement Alternat Med. 2017;2017:5034827. [PubMed ID: 29441113]. [PubMed Central ID: PMC5758950]. https://doi.org/10.1155/2017/5034827.
-
18.
Watkins CJ. Pharmacology clear and simple: A guide to drug classifications and dosage calculations. 3rd ed. Philadelphia, Pennsylvania: F.A. Davis Company; 2018. p. 389-92.
-
19.
Akinyemi KO, Smith SI, Oyefolu AO, Coker AO. Multidrug resistance in Salmonella enterica serovar typhi isolated from patients with typhoid fever complications in Lagos, Nigeria. Public Health. 2005;119(4):321-7. [PubMed ID: 15733694]. https://doi.org/10.1016/j.puhe.2004.04.009.
-
20.
Wonnacott TH, Wonnacott RJ. Statistique: Economie-gestion-sciences médecine. 4th ed. Paris: Economia; 1995.
-
21.
M V, Dhanabal SP, Rajan S; Rajendran. Pharmacodynamic and ethnomedicinal uses of weed speices in nilgiris, Tamilnadu State, India: A review. Afr J Agric Res. 2013;8(27):3505-27. https://doi.org/10.5897/ajar2013.7042.
-
22.
Omemu AM, Faniran OW. Assessment of the antimicrobial activity of lactic acid bacteria isolated from two fermented maize products-ogi and kunnu-zaki. Malays J Microbiol. 2011;7(3):124-8.
-
23.
Zhang J, Gong J, Ding Y, Lu B, Wu X, Zhang Y. Antibacterial activity of water-phase extracts from bamboo shavings against food spoilage microorganisms. Afri J Biotechnol. 2010;9(45):7710-7.
-
24.
Akinbileje FJ, Olumekun VO, Cyril-Olutayo MC, Gabriel-Ajobiewe RAO. Effect of Bambusa tuldoides cv. ventricosa leaf extracted with fermented steep liquors of maize and sorghum on some pathogenic organisms. Malays J Microbiol. 2014;10(4):280-9. https://doi.org/10.21161/mjm.62114.
-
25.
Tiwari BK, Valdramidis VP, O'Donnell CP, Muthukumarappan K, Bourke P, Cullen PJ. Application of natural antimicrobials for food preservation. J Agric Food Chem. 2009;57(14):5987-6000. [PubMed ID: 19548681]. https://doi.org/10.1021/jf900668n.
-
26.
El-Sawi S, Aboutabl E, Sleem A, Rashed K, Ragab N, Yong T. Hepatoprotective and in-vitro anti-HIV-1 effect of Alectryon tomentosus F. Muell leaf extract and its phytochemical profile. Egypt Pharmaceut J. 2015;14(3):174. https://doi.org/10.4103/1687-4315.172863.
-
27.
Talukdar P, Dutta A, Rana S, Talukdar A. Catatonia and parkinsonism as a sequelae of typhoid fever: a rare experience. BMJ Case Rep. 2013;2013. [PubMed ID: 23814211]. [PubMed Central ID: PMC3703017]. https://doi.org/10.1136/bcr-2013-010220.
-
28.
Panda A, Tatarov I, Masek BJ, Hardick J, Crusan A, Wakefield T, et al. A rabbit model of non-typhoidal Salmonella bacteremia. Comp Immunol Microbiol Infect Dis. 2014;37(4):211-20. [PubMed ID: 25033732]. [PubMed Central ID: PMC4167468]. https://doi.org/10.1016/j.cimid.2014.05.004.
-
29.
Chaudhuri D, Roy Chowdhury A, Biswas B, Chakravortty D. Salmonella typhimurium infection leads to colonization of the mouse brain and is not completely cured with antibiotics. Front Microbiol. 2018;9:1632. [PubMed ID: 30072981]. [PubMed Central ID: PMC6058050]. https://doi.org/10.3389/fmicb.2018.01632.