1. Background
Preeclampsia is a significant obstetric problem, and is usually characterized by proteinuria, hypertension and edema (1). The prevalence of preeclampsia has been reported to be 2% to 7% of all pregnancies (2). Although several studies have been performed to understand the etiology of this syndrome, the exact mechanism which leads to preeclampsia is largely unknown (3). Several investigations indicate that oxidative stress in women with preeclampsia is higher than in women with a normal pregnancy (4-6), although oxidative stress is higher in normal pregnancies than in non-pregnant women.
Soluble vascular endothelial growth factor (VEGF) receptor-1 is an anti-angiogenic protein, which acts by inhibition of VEGF and placental growth factor (PlGF), two angiogenic factors, which increase during the first two trimesters in preeclamptic women (7). The soluble FMS-like tyrosine kinase-1 (sFlt-1) levels decline 24 hours post-delivery in both preeclamptic and normal subjects. SFlt-1 is a splice variant form of a membrane bound protein that contains an extracellular domain, a trans-membrane domain and an intracellular domain (8). The splice variant mRNA for sFlt-1 has six IgG like domains. Soluble FMS-like tyrosine kinase-1 reduces the biological activity of PlGF and VEGF by blocking their interaction with Flt-1 by binding to them. The high concentrations of sFlt-1 in preeclamptic patients are thought to cause endothelial dysfunction and leads to an antiangiogenic state. Soluble FMS-like tyrosine kinase-1 administration to pregnant rats caused a reduction in blood pressure and VEGF concentrations in comparison with controls. Furthermore, basal superoxide dismutase levels and NADPH productions increased in the placenta, renal cortex and rat aorta (9). After placental perfusion was reduced, an increase in plasminogen activator inhibitor-1 (PAI-1) and decrease in plasminogen activator inhibitor-2 (PAI-2) was observed in preeclamptic patients. Hence, the ratio of PAI1: PAI2 may be a useful predictor of preeclampsia (10).
2. Objectives
The aim of this study was to investigate the effects of selenium supplementation on sFlt-1 and glutathione peroxidase (GPx) activity, and PAI1: PAI2 ratio in pregnant women at high risk of preeclampsia.
3. Materials and Methods
3.1. Subjects
Ethical approval was obtained from the Ethical Committee of Mashhad University of Medical Sciences. Two hundred and eighteen pregnant women, aged 16 to 35 years, were assessed for eligibility to participate in this trial. These subjects were randomly selected from women who had been referred to the Obstetrics and Gynecology Department of OM-Albanin and Ghaem Hospitals (Mashhad, Iran), between June 2006 and August 2008, using a random number table. The inclusion criteria were gestational age of more than 12 weeks with a live fetus and no indication for a termination of pregnancy. Exclusion criteria were consumption of any drug, except routine supplementations of folic acid and ferrous sulfate, and a history of thyroid dysfunction, diabetes, hypertension or infections. Thirty-nine subjects were excluded from the study because either they were taking medicines other than Fe and acid folic, or had diseases, which would influence the study results. The one hundred and sixty-six remaining subjects were randomly allocated into two groups using a random number table. The selenium group (n = 83) received 100 µg/day of selenium, as selenium yeast, for six month, and the placebo group (n = 83) used daily placebo yeast tablets for the same period. Thirteen pregnant women could not tolerate the supplements (n = 4) or refused to continue because of the unpleasant aroma associated with tablets (n = 9). One hundred and twenty-five subjects completed the study (61 cases in the selenium group and 64 cases in the control group) (Figure 1). Selenium yeast tablets and matching placebo yeast tablets were provided by Pharma Nord Vejle (Denmark).
3.2. Sample Collection
Blood samples were taken in the morning from each woman, after overnight fasting, in plain serum tubes for sFlt-1 and selenium measurement and in chilled tubes containing heparin for PAI-1: PAI-2 ratio and GPx measurement. Blood samples were left to clot for 30-60 minutes and then centrifuged at 2500 rpm for 15 minutes at room temperature. Serum was stored at -70°C prior to analysis.
3.3. Soluble Fms-Like Tyrosine Kinase-1 Measurement
The serum concentration of sFlt-1 was measured using commercial assays from R&D systems (Quantikine® Human Soluble VEGF R1/Flt-1 Immunoassay, Catalog number DVR100B). The sensitivity of the assay was 3.5 picograms per milliliter (pg/mL) and the intra- and inter- assay variation were 3.2% and 7.4%, respectively.
3.4. Glutathione Peroxidase Measurement
The plasma activity of GPx was measured using the glutathione assay kit (Item No.703102) from the Cayman Chemical Company. In this kit, the intra-assay coefficient of variation was 5.7% and inter assay coefficient of variation was 7.2%.
3.5. Measuring of Plasminogen Activator Inhibitor-1 and Plasminogen Activator Inhibitor-2 and Calculation of Their Ratio
Plasminogen activator inhibitor-1 was measured by the ELISA kit (Assay Max Human Plasminogen Activator Inhibitor-1 (PAI-1) from Assay Pro Company, USA). The minimum detectable concentration of PAI-1 was 200 pg/mL and the inter- and intra-assay coefficients of variation were 4.9 % and 7.1 %, respectively. This assay recognizes both natural and recombinant human PAI-1. We used the IMUBIND® assay (American Diagnostic Company Inc.) for measuring PAI-2. The intra- and inter-assay coefficients of variation (CV) for this PAI-2 ELISA kit were 5.6% and 7.8% for the high concentration (6 ng/mL) and 8.3% and 14.5% for the low concentration (1.5 ng/mL), respectively.
3.6. Statistical Analysis
Data were analyzed using the SPSS software (version 16). All data were presented as means ± standard deviation or median and inter quintile range as appropriate. Comparisons were performed by paired t-test for dependent groups and Mann-Whitney U-test for independent groups.
4. Results
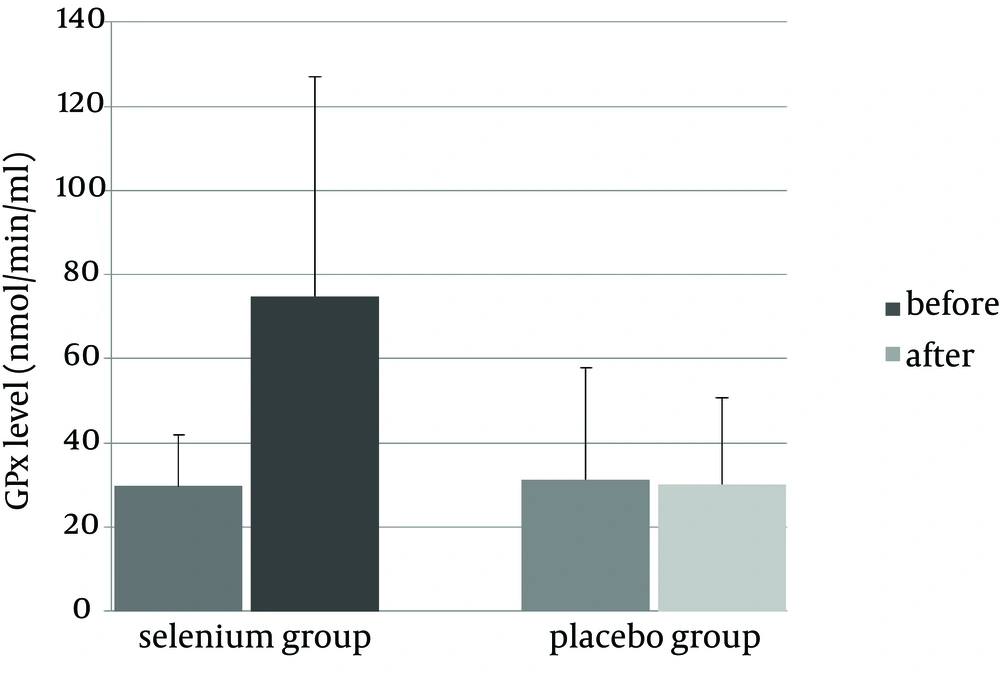
The demographic data for the selenium and placebo groups are presented in Table 1. No differences were found in age, anthropometric indices, lipid profile, past medical history (miscarriage and infertility history) or family history (incidence of preeclampsia, diabetes, hypertension and hyperlipidemia) between the two groups. There was no difference between GPx activity levels between the two groups (P = 0.08) at the beginning of the trial (Figure 2). At the end of the trial, selenium supplementation increased plasma GPx level in the selenium group (P = 0.013) yet GPx levels remained the same in the placebo group (P = 0.91).
| Parameters | Selenium Group (n = 61) | Control Group (n = 64) | P Value |
|---|---|---|---|
| Age, y | 21.6 ± 2.5 | 21.6 ± 3.4 | 0.903 |
| Weight, kg | 58.5 ± 10.2 | 56.5 ± 9.8 | 0.163 |
| Height, cm | 156.7 ± 5.8 | 156.7 ± 6.3 | 0.856 |
| BMI, kg/m2 | 23.8 ± 3.8 | 23.0 ± 4.0 | 0.168 |
| Waist circumference, cm | 77.1 ± 11.6 | 4.9 ± 8.2 | 0.132 |
| Hip circumference, cm | 95.8±12.1 | 94.8 ± 7.9 | 0.500 |
| Waist: hip ratio | 0.8 ± 0.08 | 0.8 ± 0.06 | 0.868 |
| SBP, mmHg | 10.49 ± 100.14 | 11.41 ± 101.86 | 0.269 |
| DBP, mmHg | 61.62 ± 8.2 | 70.16 ± 9.17 | 0.778 |
| Chol, mg/dL | 60.16 ± 177.25 | 53.4 ± 165.88 | 0.968 |
| TG, mg/dL | 34.67 ± 89.18 | 34 ± 88.88 | 0.348 |
| LDL, mg/dL | 54.98 ± 113.84 | 49.85 ± 104.44 | 0.4 |
| HDL, mg/dL | 10.37 ± 48.76 | 8.56 ± 46.77 | 0.328 |
| Education Less than 12 years | 43.9 | 42.2 | 0.887 |
| Education up to 12 years | 50.5 | 53.3 | 0.887 |
| Education more than 12 years | 5.6 | 4.4 | 0.887 |
| History of diabetes | 1.9 | 5.5 | 0.17 |
| History of hypertension | 5.6 | 5.5 | 0.93 |
| History of hyperlipidemia | 4.7 | 5.5 | 0.79 |
a Abbreviations: BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; Chol, cholesterol; TG, Triglyceride; LDL, Low-density lipoprotein; HDL, High-density lipoprotein.
b Data are expressed as mean ± SD or %.
c No Significant difference exists between the groups (P > 0.05).
No significant difference was observed between serum sFlt-1 levels of the two groups at the beginning of the study (Table 2; P = 0.66) yet in both groups, sFlt-1 levels were increased at the end of the trial (P < 0.001). However, this increase was not significantly different between the two groups (P = 0.51). Serum PAI1 and PAI2 concentrations were significantly different before and after the trial (P < 0.001). The ratio of PAI1: PAI2 had decreased in the case group while it was elevated in the control group, but these changes were not statistically significant.
| Selenium Group | Placebo Group | P Value | |||
|---|---|---|---|---|---|
| Pre Trial | Post Trial | Pre Trail | Post Trail | ||
| Selenium, µg/dL | 122.5 ± 32. 2 | 168. 6 ± 36.4 | 122. 9 ± 26. 9 | 119.4 ± 33.4 | < 0.001 |
| sFlt-1, pg/mL | 1522 ± 518 | 5985 ± 3232 | 1462 ± 462 | 6371 ± 2306 | 0.51 |
| PAI-1, ng/mL | 54.53 ± 82.94 | 233.37 ± 311.39 | 26.25 ± 29.68 | 138.82 ± 132.26 | 0.883 |
| PAI-2, ng/mL | 27.83 ± 19.94 | 223.17 ± 195.89 | 42.32 ± 47.65 | 210.46 ± 217.87 | 0.461 |
| PAI-1/PAI-2 | 1.2 (0.23-94.53) | 0.44 (0.99-91.1) | 0.68 (0.08-5.24) | 0.96 (0.07-91.10) | 0.443 |
a Abbreviations: PAI-1, plasminogen activator inhibitor1; PAI-2, plasminogen activator inhibitor2; sFlt-1, soluble FMS like tyrosine kinase-1.
b Data are expressed as mean ± SD (for normally distributed data) or median and interquartile range (for non-normally distributed data). Compression between pre- and post-trail values was made using paired t-test for normally distributed data or Wilcoxon test for non-normally distributed data.
5. Discussion
Selenium supplementation was found to increase plasma GPx activity and reduce the PAI1: PAI2 level in the selenium-supplemented group compared to the control group, but it had no significant effect on serum sFlt-1 level. Increasing the plasma GPx activity in the selenium group while the GPx level in placebo group remains unchanged is a finding that is consistent with our previous study, which indicated a reduction in selenium during preeclampsia and showed selenium supplementation increases the serum selenium level in mothers who consume selenium tablets (11), as selenium can change the pattern of selenoprotein expression (12). Furthermore, PAI-1 and PAI-2 levels increased during pregnancy in both the placebo and selenium groups. Chapell and colleagues reported that the ratio of PAI1: PAI2 decreased in pregnant women at low risk of preeclampsia during pregnancy and this ratio increased in pregnant women who developed preeclampsia (10). Other studies (13-15) have also found a higher PAI1: PAI2 ratio in women with preeclampsia compared to healthy women, and this ratio was proposed as a predictive marker of preeclampsia in these studies, yet changes in the PAI1: PAI2 ratio also occurs during normal pregnancy. In some studies it was found that PAI1: PAI2 ratio is reduced during normal pregnancy, while the results of other studies (13, 14) demonstrated no significant change in this ratio in normal pregnancies. Our data can be interpreted in two ways. The ratio of PAI1: PAI2 in the selenium group at the beginning of the study was higher than the placebo group, but became less than in the placebo group by the end of the study Selenium treatment may influence the PAI1: PAI2 ratio, and reduce oxidative stress.
Many studies have now shown that sFlt-1 may be responsible for the clinical manifestation of preeclampsia. For example, Reddy et al. reported that serum sFlt-1 levels were higher pre-labor/pre-delivery in preeclamptic pregnancies than in normal pregnancies (16). Furthermore, in preeclamptic women, labor increased the levels of sFlt-1 (16). In another study the levels of sFlt-1 and PlGF were compared in 46 preeclamptic and 100 normal pregnant women. The PIGF levels were significantly lower in the preeclamptic women than in normal controls, while the sFlt-1 levels were significantly higher. The sFlt-1: PlGF ratio was significantly higher in preeclamptic women than in normal controls leading the authors to propose that the sFlt-1: PlGF ratio may be an early predictive marker of subsequent development of preeclampsia (17). These studies (16, 17) are consistent with our findings, yet selenium supplementation was not associated with a decrease in sFlt-1 level. Since arterial changes occur during the first trimester, and antioxidants may influence sFlt-1 expression early on, it maybe that antioxidant supplementation may have an effect if started in the first trimester of pregnancy or even before gestation. The limitations of our study, include a relatively small sample size and short period of treatment, thus further studies may be required with a larger sample size with supplementation started from the beginning of the gestation period.
Furthermore, in our sample population the baseline serum selenium concentrations were not low; thus the effects of selenium supplementation maybe greater in regions with lower selenium status. Overall, selenium administration in the second and third trimesters of pregnancy significantly raised the antioxidant level and improved the PAI: 1lPAI2 ratio though not to a significant extent. There was no effect on serum sFlt-1 levels though this might be a consequence of the relatively short period of selenium supplementation in this study.