Abstract
Background:
Intraventricular hemorrhage (IVH) is a common complication seen in premature infants. Since the brain intraventricular hemorrhage in any degree of risk is an important factor in long-term neuropathology, the role of magnesium sulfate on cerebral hemorrhage requires further investigation. Therefore, this study aimed to investigate the effect of magnesium sulfate on intraventricular hemorrhage in infants of mothers with premature rupture of membranes.Methods:
This study is a double blind clinical trial (IRCT: IRCT2016080729223N1) on 120 pregnant women with premature rupture of membranes at 34 weeks admitted to the hospital of Shahid Akbar Abadi that were selected based on the inclusion criteria and assigned to two groups (magnesium sulfate recipients and non-recipients). The significance level was set at p<0.05.Results:
The mean age was 28.17 ± 6.21 in the intervention group and 28.33 ± 5.97 in the control group that showed no statistically significant difference between the groups. The average weight of infants in the intervention group and in the control group was 2336.1 ± 526.8 and 1975.3 ± 233.4, respectively, which showed no significant difference. No intraventricular brain hemorrhage occurred in the infants of the two groups.Conclusions:
Magnesium sulfate needs more evaluation in prevention of intraventricular hemorrhage in infants of mothers with premature rupture of membranes at 34 weeks.Keywords
1. Background
Intraventricular hemorrhage (IVH) is a common complication seen in low birth weight neonates. Preterm rupture of membranes before labor and earlier than 37 weeks of age is among the contributing factors to preterm birth. Prematurity and low birth-weight are two important risk factors for IVH. Factors such as respiratory distress, hypoxia-related injury, ischemia, blood pressure decrease or increase, increased venous pressure, pneumothorax, and hypovolemia would increase the probability of IVH. This side effect may occur in the first 72 hours of birth; half of the cases are seen in the first day of life, and more than 90% of cases may be seen up to end of the first week (1-4). IVH and periventricular hemorrhage are seen in 50% of VLBW neonates and in those younger than 35 weeks in the United States although the rate recently has decreased. It is a common condition in preterm neonates born before 32 weeks. Nevertheless, it may also be seen in higher gestational ages or in term neonates (5, 6). Half of the neonates born before 34 weeks and only 4% of term neonates have IVH records (7). IVH may result in prolonged disability, cerebral palsy, mental retardation, seizure, behavioral and cognitive problems, and death (8-10). Most cerebral bleeding cases are seen in germinal matrix that has high blood supply and prone to bleeding (9-12). The anatomical conditions may result in venous congestion and stasis leading to increased intravascular pressure and ruptured vessels. The auto regulation is abnormal in preterm (7, 9, 12, 13). Prematurity and lack of development of brain vessels may result in bleeding by small stimulations (14). Symptoms of IVH are unspecific and are usually related to severity of disease. In acute, severe cases, sudden change to bad health status, severe sudden pale skin, acute anemia, respiratory disorder, and fontanel bulging may occur.
Currently, brain ultrasonography or magnetic resonance imaging is used in the first three days of life and in suspected cases, it is repeated two or three times to assess the severity (15-17). The bleeding from germinal matrix around the brain ventricle is subdivided according to IVH extension in brain ultrasonography into four categories of grade 1 to 4: grade 1 limited bleeding to germinal matrix; grade 2 Intraventricular hemorrhage; grade 3 bleeding with ventricular extension; and grade 4 extension of bleeding to brain parenchyma. Grades 1 and 2 are removed spontaneously without sequel while grades 3 and 4 are accompanied with severe sequels (10). Since PROM and preterm birth are main etiologies of premature neonates, different methods are used to prevent preterm labor. Tocolytic treatments are among the conventional methods but the best therapeutic method is controversial. Magnesium sulfate, prostaglandin inhibitors, calcium channel blockers, and nitric oxide releasing drugs are among those with positive effects. Magnesium sulfate is a tocolytic method of prevention from preterm labor. However, there are scarce documents about beneficial effects of this drug on the improvement of pregnancy outcomes in various gestational ages; it is the first-line treatment in many centers. The objective of the use of tocolytic is to delay the active phase of labor (9, 10, 17, 18). Epidemiological documents have shown that treatment of mothers with magnesium sulfate would result in myocardial stability and blood supply in placenta and fetal brain (19), and reduction of ischemic region (20) and anti-oxidant effects with decreased platelet adhesion (21) are neuroprotective in fetus. Nevertheless, the results of studies are controversial. Marret et al. in 2008 demonstrated that the use of magnesium sulfate would significantly reduce brain dysfunction (22). In addition, another multi-center clinical trial by Dwight et al. in 2008 assessed the effect of magnesium sulfate on the prevention of cerebral palsy. It was seen that moderate and severe forms of cerebral palsy significantly reduced in patients who received magnesium sulfate; but the mortality rate in those with cerebral palsy did not decrease (23).
The study by Carlo Gian et al. in 2005 revealed that the use of aminophylline and magnesium sulfate was accompanied by decreased Intraventricular hemorrhage in neonates under 30 weeks’ gestational age (24). However, the study by Nakazawa and colleagues in 2015 showed that tocolytic therapy with magnesium sulfate would result in increased risk of death, neurological pathologies, and Intraventricular hemorrhage in neonates (25). In addition, Petrova et al. in 2012 reported no association of prenatal administration of magnesium sulfate with Intraventricular hemorrhage and brain parenchyma injuries in neonates (26). Hence, regarding the extended use of magnesium sulfate in health care centers of our country, the outcomes of IVH in premature neonates were assessed in this clinical trial.
2. Methods
In this double-blind randomization clinical trial (IRCT: IRCT2016080729223N1) with local ethical registration code of IR.IUMS.rec.1394.9111290015, pregnant women with premature rupture of membranes at 34 weeks’ gestational age attending to Akbarabadi hospital, Tehran, Iran, were enrolled after signing an informed consent form. According to the formula for proportion estimation in two group (incidence of IVH) by assumption of 95% confidence interval, alpha amount of 5%, beta amount of 20%, and incidence rate of 27% (27), two groups of 60 pregnant women who had rupture of membranes in ultrasonography at 34 weeks’ gestational age were enrolled.
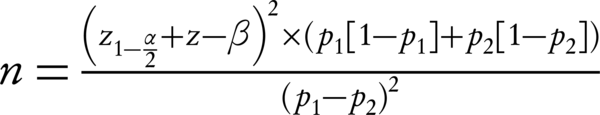
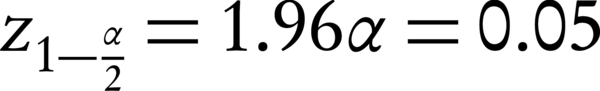
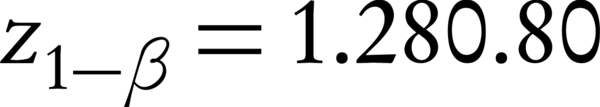
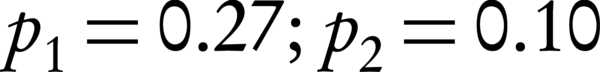
The exclusion criteria were lack of hypertension, preeclampsia, trauma, gestational or aggravated diabetes, any type of metabolic disease affecting the pregnancy outcome, long-term drug use, and gestational histories affecting the pregnancy outcomes (Figure 1).
CONSORT Flow Diagram for Study Design.
Participants were randomly allocated to either the two groups by means of a computer-generated randomization list and by using sealed opaque medication packets that numbered and used consecutively. Beside the conventional treatments, the intravenous magnesium sulfate was administered in the intervention group at dose of 6 g during 20 to 30 minutes, followed by 2 g per hour during 12 hours before labor. Patients were excluded if major side effects were seen such as hypotension, hyporeflexia, flaccid paresis, heart block, respiratory paresis, infusion site pain, and hypothermia. In the control group, conventional treatment with normal saline infusion was used. Finally, the neonates in both groups were assessed for IVH, and cranial ultrasonography was requested if necessary. Otherwise, they all were assessed with ultrasonography by an experienced radiologist who was blind about the groups at 3rd and 7th day. MRI was done in suspected cases. In addition, the lab sample was sent for all patients. Demographic data were gathered for all cases. Finally, data analysis was performed by SPSS16 software with Chi-Square and T test or nonparametric parallel tests.
3. Results
In this randomized clinical trial, the mean age was 28.17 ± 6.21 and 28.33 ± 5.97 years in the intervention and control groups, respectively (P = 0.880). The mean birth weight was 1975.3 ± 233.4 and 2336.1 ± 526.8 g in intervention and control groups, respectively, without any significant difference by T test (P = 0.273). The mean Apgar was 8.42 ± 0.74 and 8.47 ± 0.62 g in the intervention and control groups, respectively, with no significant difference by T test (P = 0.690) (Table 1). ICU stay was 1.4 ± 0.62 and 1.38 ± 0.45 gram in the intervention and control groups, respectively, with no significant difference by T test (P = 0.907). Twin and multiple pregnancies and stillbirth were not seen. 27 subjects (45%) in the intervention group were male and 33 (55%) were female. The CRP was negative in 56 subjects (93.3%) and positive in 4 patients (6.7%) in the intervention group. The platelet, sodium, potassium, calcium, BS, and ICU stay are compared by T test as presented in Table 2. As shown in Table 3, the rate of IVH as the main outcome was 5% and 1.6% in the intervention and control groups, respectively (P = 0.981).
Characteristics of Participants in the Two Groups
| Variable | Intervention Group (n = 60) | Control Group (n = 60) | P Value | CI 95 % | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Age | 28.17 ± 6.21 | 28.33 ± 5.97 | 0.880 | -2.35 | 2.02 |
| Wight | 1975.3 ± 233.4 | 2336.1 ± 526.8 | 0.273 | -1009.5 | 287.9 |
| Apgar | 8.42 ± 0.74 | 8.47 ± 0.62 | 0.690 | -0.298 | 0.198 |
| WBC | 11300 ± 10200 | 80200 ± 12800 | 0.038 | 0.187 | 6.376 |
| HB | 15.1 ± 1.20 | 15.6 ± 1.10 | 0.026 | -0.894 | -0.058 |
Laboratory Results in the Two Groups
| Variable | Intervention Group (n = 60) | Control Group (n = 60) | P Value | CI95% | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Platelet | 238.2 ± 55.5 | 247.9 ± 34.6 | 0.255 | -26.53 | 7.11 |
| Hemoglobin | 15.1 ± 1.20 | 15.6 ± 1.10 | 0.026 | -0.894 | -0.058 |
| Leukocyte | 11300 ± 1020 | 8020 ± 1280 | 0.038 | 0.187 | 6.376 |
| Sodium | 141.8 ± 2.15 | 141.2 ± 2.04 | 0.155 | -0.211 | 1.311 |
| Potassium | 4.25 ± 0.18 | 4.25 ± 0.23 | 0.069 | -0.070 | 0.083 |
| Calcium | 8.99 ± 0.57 | 9.07 ± 0.39 | 0.036 | -0.259 | 0.096 |
| BS | 70.85 ± 12.25 | 12.51 ± 78.48 | 0.001 | -12.11 | -3.15 |
| NIW | 1.40 ± 0.62 | 1.38 ± 0.61 | 0.907 | -0.212 | 0.238 |
IVH of the Two Groups
| IVH Grade I | IVH Grade II | IVH Grade III | IVH Grade IV | P Value | |
|---|---|---|---|---|---|
| Intervention group (n = 60) | 2 (3.3) | 1 (1.6) | 0 (0) | 0 (0) | 0.981 |
| Control group (n = 60) | 0 (0) | 1 (1.6) | 0 (0) | 0 (0) |
4. Discussion
Preterm birth is a main etiology of premature neonates, which is related to brain hemorrhage and neuropathic lesions. Different strategies are used to prevent preterm labor. For example, the magnesium sulfate would result in decreased IVH rate but it is controversial. However, there are scarce documents about useful effects of this drug on the improvement of pregnancy outcomes in various gestational ages; it is the first-line treatment in many centers. Some studies have shown that magnesium sulfate may decrease the neurological injury rates leading to cerebral palsy although opposite results also are reported. Since IVH in every grade is a risk factor for long-term neuropathology, the role of magnesium sulfate should be assessed. This study was carried out to evaluate the effect of magnesium sulfate on the prevention of IVH in neonates of mothers with PROM. The results demonstrated that the main outcome, i.e. IVH, was not differed between the two groups comprising neonates receiving and not-receiving magnesium sulfate. These findings are in congruence with the findings of Dwight J. Rose et al. in 2008 (28). In their study, magnesium sulfate had no significant effect on prevention of moderate to severe cerebral palsy. In a randomized clinical trial conducted by Crowther et al. (29) among 1062 pregnant women with 30 weeks’ gestational age, magnesium sulfate had no effect on the prevention of cerebral palsy and injury. However, mortality rate before two years of age decreased significantly by magnesium sulfate. Other randomized clinical trials about this drug had shown different results. This may be due to different design, sample size, or follow-up patterns. The study by Papile et al. (10) demonstrated that the use of magnesium sulfate would result in decreased death rate. The study by Petrova et al. in 2012 revealed that there is no significant association between the use of this drug and IVH and parenchyma injury. However, in their study, patients with hypertension and preeclampsia were enrolled, leading to increased use of magnesium sulfate (26). Gain Carlo et al. in 2005 revealed that aminophylline and magnesium sulfate would result in less IVH in neonates under 30 weeks but it had no effect on respiratory distress syndrome, patent ductus arteriosus, and retinopathy (24).
Due to anti-arrhythmia, neuroprotective effects, and ischemia reduction (30), the significant effect on IVH was expected. Nevertheless, the lack of effect may be due to lower rates of IVH compared to worldwide statistics, leading to lower positive samples that may affected the results. Low sample size may increase the chance of effect by higher random error. It is likely that the ultrasonography method and diagnostic sensitivity were less than expected. The limited use of MRI may be effective in the sensitivity reduction, as well. The follow-up duration may also be effective. Hence, studies with larger sample sizes, and more specific methods, and longer follow-up periods are recommended.
Also, comparison of mean birth weight, Apgar, leukocyte count, hemoglobin, platelet, sodium, potassium, BS, and ICU stay between the two groups and comparison of CRP, delivery method, and sex of neonate between the two groups revealed that only BS, leukocyte count, and hemoglobin showed significant differences.
References
-
1.
Christian EA, Jin DL, Attenello F, Wen T, Cen S, Mack WJ, et al. Trends in hospitalization of preterm infants with intraventricular hemorrhage and hydrocephalus in the United States, 2000-2010. J Neurosurg Pediatr. 2016;17(3):260-9. [PubMed ID: 26544084]. https://doi.org/10.3171/2015.7.PEDS15140.
-
2.
Jantzen C, Lodha A, Lucia M, Lee SK, Ye XY, Sankaran K. Effects of nosocomial infection trends on neonatal outcomes in preterm infants <33 weeks of gestational age in Canadian NICUs. Zhongguo Dang Dai Er Ke Za Zhi. 2015;17(10):1019-27. [PubMed ID: 26483217].
-
3.
Chaichian S, Akhlaghi A, Rousta F, Safavi M. Experience of water birth delivery in Iran. Arch Iran Med. 2009;12(5):468-71. [PubMed ID: 19722768].
-
4.
Motasaddi-Zarandy M, Rezai H, Mahdavi-Arab M, Golestan B. The scholastic achievement of profoundly deaf children with cochlear implants compared to their normal peers. Arch Iran Med. 2009;12(5):441-7. [PubMed ID: 19722764].
-
5.
Mehdizadeh A, Roosta F, Chaichian S, Alaghehbandan R. Evaluation of the impact of birth preparation courses on the health of the mother and the newborn. Am J Perinatol. 2005;22(1):7-9. [PubMed ID: 15668838]. https://doi.org/10.1055/s-2004-837738.
-
6.
Motamedi N, Goodarzi E, Rahimi Pordanjani S, Valizadeh R, Moradi Y, Sohrabivafa M, et al. Incidence of phenylketonuria in Lorestan province, West of Iran (2006-2016). Int J Pediatr. 2017.
-
7.
Horsager R, Roberts S, Rogers V, Santiago-Mu-oz P, Worley K, Hoffman B. Williams Obstetrics, Study Guide. McGraw Hill Professional; 2014.
-
8.
Kadri H, Mawla AA, Kazah J. The incidence, timing, and predisposing factors of germinal matrix and intraventricular hemorrhage (GMH/IVH) in preterm neonates. Childs Nerv Syst. 2006;22(9):1086-90. [PubMed ID: 16636880]. https://doi.org/10.1007/s00381-006-0050-6.
-
9.
Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92(4):529-34. [PubMed ID: 305471].
-
10.
Papile LA, Munsick-Bruno G, Schaefer A. Relationship of cerebral intraventricular hemorrhage and early childhood neurologic handicaps. J Pediatr. 1983;103(2):273-7. [PubMed ID: 6875724].
-
11.
Haghighi L, Nohesara S, Moradi Y, Rashidi M, Moridi M. Psychological Disorders in Women with Spontaneous Preterm Delivery. Shiraz E-Med J. 2016;17(10).
-
12.
Moradi Y, Baradaran HR, Yazdandoost M, Atrak S, Kashanian M. Prevalence of Burnout in residents of obstetrics and gynecology: A systematic review and meta-analysis. Med J Islam Repub Iran. 2015;29(4):235. [PubMed ID: 26793673].
-
13.
Haghighi L, Hashemi N, Moradi Y, Barzegar N, Najmi Z. The Association Between Menarche Age and First Offspring Sex Ratio. Shiraz E-Med J. 2016;17(1).
-
14.
Bada HS, Hajjar W, Chua C, Sumner DS. Noninvasive diagnosis of neonatal asphyxia and intraventricular hemorrhage by Doppler ultrasound. J Pediatr. 1979;95(5 Pt 1):775-9. [PubMed ID: 490249].
-
15.
Ahmann PA, Lazzara A, Dykes FD, Brann AJ, Schwartz JF. Intraventricular hemorrhage in the high-risk preterm infant: incidence and outcome. Ann Neurol. 1980;7(2):118-24. [PubMed ID: 7369717]. https://doi.org/10.1002/ana.410070205.
-
16.
Bada HS, Korones SB, Perry EH, Arheart KL, Ray JD, Pourcyrous M, et al. Mean arterial blood pressure changes in premature infants and those at risk for intraventricular hemorrhage. J Pediatr. 1990;117(4):607-14. [PubMed ID: 2213390].
-
17.
Perlman JM, Goodman S, Kreusser KL, Volpe JJ. Reduction in intraventricular hemorrhage by elimination of fluctuating cerebral blood-flow velocity in preterm infants with respiratory distress syndrome. N Engl J Med. 1985;312(21):1353-7. [PubMed ID: 3887165]. https://doi.org/10.1056/NEJM198505233122104.
-
18.
Stark MJ, Hodyl NA, Andersen CC. Effects of antenatal magnesium sulfate treatment for neonatal neuro-protection on cerebral oxygen kinetics. Pediatr Res. 2015;78(3):310-4. [PubMed ID: 25985294]. https://doi.org/10.1038/pr.2015.96.
-
19.
Mittendorf R, Pryde PG. An overview of the possible relationship between antenatal pharmacologic magnesium and cerebral palsy. J Perinat Med. 2000;28(4):286-93. [PubMed ID: 11031698]. https://doi.org/10.1515/JPM.2000.035.
-
20.
Garnier Y, Middelanis J, Jensen A, Berger R. Neuroprotective Effects of Magnesium on Metabolic Disturbance in Fetal Hippocampal Slices After Oxygen-Glucose Deprivation: Mediation By Nitric Oxide System. J Soc Gynecol Investigat. 2016;9(2):86-92. https://doi.org/10.1177/107155760200900207.
-
21.
Elimian A, Verma R, Ogburn P, Wiencek V, Spitzer A, Quirk JG. Magnesium sulfate and neonatal outcomes of preterm neonates. J Matern Fetal Neonatal Med. 2002;12(2):118-22. [PubMed ID: 12420842]. https://doi.org/10.1080/jmf.12.2.118.122.
-
22.
Marret S, Benichou J. Antenatal magnesium sulfate and outcomes for school-aged children. JAMA. 2015;313(3):306. [PubMed ID: 25603006]. https://doi.org/10.1001/jama.2014.15912.
-
23.
Hirtz DG, Weiner SJ, Bulas D, DiPietro M, Seibert J, Rouse DJ, et al. Antenatal Magnesium and Cerebral Palsy in Preterm Infants. J Pediatr. 2015;167(4):834-839 e3. [PubMed ID: 26254839]. https://doi.org/10.1016/j.jpeds.2015.06.067.
-
24.
Di Renzo GC, Mignosa M, Gerli S, Burnelli L, Luzi G, Clerici G, et al. The combined maternal administration of magnesium sulfate and aminophylline reduces intraventricular hemorrhage in very preterm neonates. Am J Obstet Gynecol. 2005;192(2):433-8. [PubMed ID: 15695983]. https://doi.org/10.1016/j.ajog.2004.07.078.
-
25.
Nakazawa H, Uchida A, Minamitani T, Makishi A, Takamatsu Y, Kiyoshi K, et al. Factors affecting maternal serum magnesium levels during long-term magnesium sulfate tocolysis in singleton and twin pregnancy. J Obstet Gynaecol Res. 2015;41(8):1178-84. [PubMed ID: 25857633]. https://doi.org/10.1111/jog.12690.
-
26.
Petrova A, Mehta R. Magnesium sulfate tocolysis and intraventricular hemorrhage in very preterm infants. Indian J Pediatr. 2012;79(1):43-7. [PubMed ID: 21625843]. https://doi.org/10.1007/s12098-011-0440-y.
-
27.
Canterino JC, Verma UL, Visintainer PF, Figueroa R, Klein SA, Tejani NA. Maternal magnesium sulfate and the development of neonatal periventricular leucomalacia and intraventricular hemorrhage. Obstet Gynecol. 1999;93(3):396-402. [PubMed ID: 10074987].
-
28.
Rouse DJ, Hirtz DG, Thom E, Varner MW, Spong CY, Mercer BM, et al. A randomized, controlled trial of magnesium sulfate for the prevention of cerebral palsy. N Engl J Med. 2008;359(9):895-905. [PubMed ID: 18753646]. https://doi.org/10.1056/NEJMoa0801187.
-
29.
Grether JK, Hoogstrate J, Walsh-Greene E, Nelson KB. Magnesium sulfate for tocolysis and risk of spastic cerebral palsy in premature children born to women without preeclampsia. Am J Obstet Gynecol. 2000;183(3):717-25. [PubMed ID: 10992199]. https://doi.org/10.1067/mob.2000.106581.
-
30.
Ravn HB, Moeldrup U, Brookes CI, Ilkjaer LB, White P, Chew M, et al. Intravenous magnesium reduces infarct size after ischemia/reperfusion injury combined with a thrombogenic lesion in the left anterior descending artery. Arterioscler Thromb Vasc Biol. 1999;19(3):569-74. [PubMed ID: 10073959].