1. Background
Kidney transplantation is a promising choice of treatment in patients with end-stage renal disease (ESRD). The role of Metabolic conditions such as diabetes and abnormal lipid profile and cardiovascular events on the long-term patient and graft survival has been noticed before (1-4). Diabetes mellitus (DM) is an independent predictor of major cardiovascular events after transplantation, and serum glucose control strongly correlated with graft function and survival post-transplantation (2, 5). Oxidative stress plays a key role in the pathogenesis of diabetes and dyslipidemia complications, including vascular disease, nephropathy, retinopathy, and even neuropathy associated with diabetes (6). Oxidative stress is defined as the state of amplified reactive oxygen species (ROS) and/or decreased intrinsic antioxidant support (7). Disturbed lipid regulation and chronic hyperglycemia are the main sources of this phenomenon, which is commonly seen in diabetes (8). Although oxidative stress has a critical role in diabetes complications, antioxidants, and vitamins have either failed to show any long-term benefits or have produced inconsistent results. Accordingly, there has been growing attention for the potential roles of oral insulin-sensitizing agents, including Pioglitazone, to reduce oxidative stress (9). Pioglitazone is a member of which has a high affinity for peroxisome proliferator-activated receptor gamma (PPARγ), increase the sensitivity of insulin receptors is currently used in the treatment of DM (10). Nephroprotection in diabetic nephropathy and anti-inflammatory properties were reported before by this medication (11, 12). In previous studies, pioglitazone shows potent antioxidant properties (13). PPARγ decreases the inflammation by reducing the inflammatory mediator’s production by preventing the activation of transcription factors (12). Malondialdehyde (MDA) and total protein carbonyls (TPC) content measure reactive aldehyde species as indices of oxidative stress (14). Reactive oxygen species (ROS) as an important mediator of cellular damage could cause lipid peroxidation which stands for the most important expression of ROS-induced oxidative stress (15). Determination of carbonyl level is used as an index of the extent of the oxidative damage of protein, while the MDA level is a marker of lipid oxidation (16). Oxygen radicals cause lipid peroxidation of cell and organelle membranes, disrupting the structural integrity and capacity for cell transport and energy production (17). In time post-transplantation patients could go through the different status of oxidative stress expressing potential changes induced by any of the causes of graft dysfunction (18). It was studied before that after transplantation and restoring kidney function, oxidative stress could reduce over time. Also, increased systemic biomarkers of oxidative stress in kidney transplant recipients, especially in the early phase and after that could lead to chronic rejection (19, 20). We also observed pioglitazone effects on kidney transplant recipients’ blood glucose and inflammatory markers previously (21).
2. Objectives
In the following of the study, in this clinical trial, our goal was to evaluate the effect of pioglitazone on oxidative stress biomarkers, including MDA and TPC in this group of patients at triple-blind, randomized, placebo-controlled trial.
3. Methods
3.1. Study Design
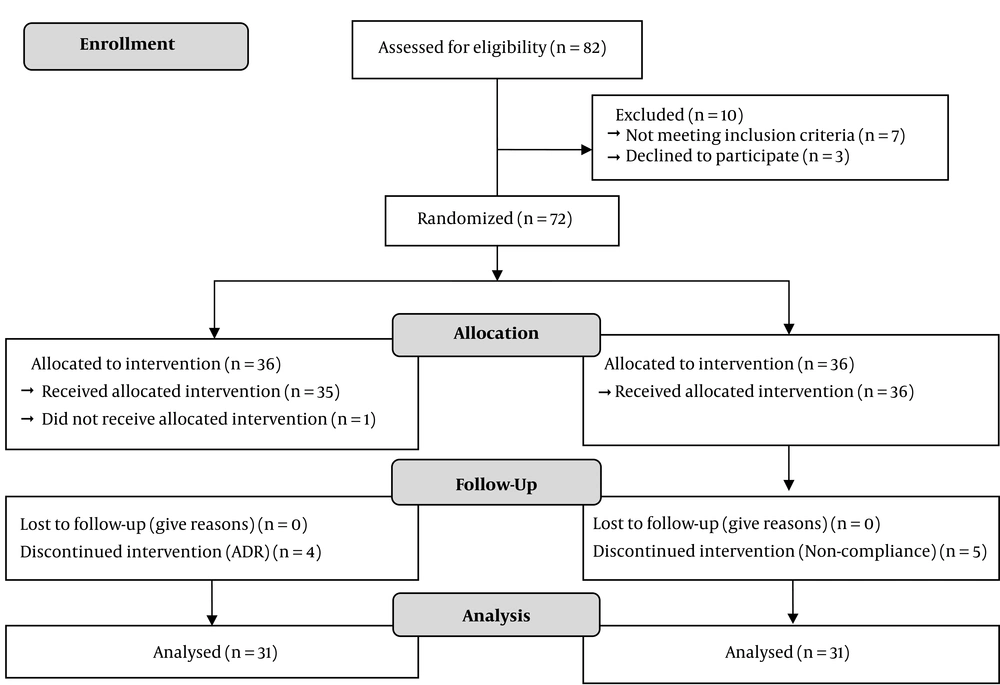
We conducted a triple-blind randomized placebo-controlled trial to evaluate the effects of Pioglitazone on HBA1c and oxidative stress biomarkers, MDA, and TPC levels in diabetic kidney transplant recipients. Inclusion and not-inclusion criteria were as followed. Diabetic kidney transplant recipients with more than one-month post-transplant time with at least 14 days of stable graft function before enrollment in Shahid Labbafinejad Medical Centre were recruited for the study from September 2012 to May 2013. Patients were not included if they had Glomerular filtration rate (GFR) of 30 mL/min or less based on Cockcroft-Gault equation at the baseline (22), Congestive heart failure (CHF) class II-IV based on New York Heart Association (NYHA) classification (23), history of fever episodes, history of hepatitis B or C and pregnancy during the study. Patients who had an episode of acute allograft dysfunction (decreased GFR at least 30 mL/min) or received corticosteroid pulse therapy during the follow-up were excluded from the study. All donors and recipients were analyzed for compatibility by performing the matching process before transplantation in our center. After assessing patient’s eligibility for enrollment Eighty-two diabetic kidney transplanted patients recruited and ten patients were excluded from the study. Finally 72 patients who received a graft from cadaveric (24%) and living (76%) donors, randomized for enrolling the trial. The random allocation sequence was computer generated and consisted of a series of group numbers (either 1 = A or 2 = B) for each consecutive patient. a block randomization method was used and each block was of 10 patients. Patients divided into two groups of control to receiving a placebo and intervention to receive 30 mg Pioglitazone per day. During the study, the medication administrator, patient, and the statistical analyzer were blind to their assessment and the concealment of randomization was adhered to. Insulin therapy for each patient continued irrespective of the group that they were assigned. Both Pioglitazone (Osve pharmaceutical company, Glutazone®, Tehran, Iran) and placebo were manufactured by the same company. In the 4 months of follow-up HBA1c, MDA, and TPC data were recorded (Figure 1). The study was carried out per principles of the Declaration of Helsinki and was approved by the ethical institutional committee of Shahid Labbafinejad Medical Centre. This trial was registered in the Iranian Registry of Clinical Trial (by the code of IRCT2012121811807N1). Written informed consent was taken from all patients included in the study. Patients visited and closely observed monthly, regularly, and also followed up by telephone for 4 months. At each visit, data were gathered for the outcome events, compliance, and side effects. All clinical and laboratory variables were documented by the supervision of a clinical pharmacist. The dose of insulin adjusted based on American Diabetes Association (ADA) guideline. According to ADA guidelines, pre-prandial plasma glucose 90 to 130 mg/dL and HBA1c < 7%, were considered as treatment goals for adults with diabetes. This study was granted from the Deputy of Research and Technology of Shahid Beheshti University of Medical Sciences.
3.2. Laboratory Studies
We measured concentrations of MDA, TPC, and HBA1c at the beginning of the study and the end of 4 months of the follow-up period. In each visits 5 mL whole blood was collected to assay these parameters. After centrifuging the samples and measurement of the mentioned parameter, the serum component was separated and frozen at -20ºC for measurements of MDA and TPC levels with the spectrophotometric method at the initiation and the end of the study.
3.3. Outcomes
The primary study outcomes were changes in oxidative stress markers in the serum, including MDA and TPC. Blood glucose control and secondary also were recorded by screening the Hb1Ac.
3.4. Statistical Analysis
All of the analyses were carried out by the Statistical Package for the Social Sciences (SPSS) version 20 software (IBM Company, New York, NY, United States). Results are expressed as means ± standard deviation (SD) or as proportions. Differences in the categorical data were analyzed by chi-square test or Fisher’s exact test was performed (if more than 25% of the categories have frequencies below five). The t-test was used for parametric data when normal distribution and equal dispersion were recognized. The Mann-Whitney U test and the Wilcoxon’s signed-rank test were used when the variance was unequal. Given that to compare the two groups which each group ranged consecutive measurements was determined by the preferred method, Repeated measure ANOVA. A P value of less than 0.05 was considered statistically significant.
4. Results
In the follow-up period, one patient in the Pioglitazone group and five in the control group excluded from the study due to non-adherence to study protocol. Also, four dropouts in the Pioglitazone group due to side effects. As it is shown in Tables 1 and 2, the differences between clinical characteristics and demographics of patients at baseline were not significantly different between the two groups. post-transplantation duration did not differ statistically significant. None of the patients in each group was a smoker. Transplantation was performed because of kidney failure due to different reasons, and patients were using insulin because of diabetes mellitus. There were no significant differences in the various medication’s consumption dose (such as Angiotensin-converting enzyme inhibitors (ACEIs), Angiotensin receptor blockers (ARBs), aspirin, allopurinol, stains, vitamin E or C, insulin, cyclosporine, and prednisolone) except for Pioglitazone in the two groups (Table 1). Ten out of 31 patients in the intervention group and 7 out of 31 patients of the control group were received a cadaveric graft. At baseline, no significant differences in blood glucose parameters, kidney function status, and inflammatory mediator levels between the two groups were observed. Changes in parameters after intervention in two groups are shown in Table 3. Mean HBA1c at baseline in the Pioglitazone group was 8.6 ± 1.9, and improvement in serum glucose control was shown in the Pioglitazone group. Tracking the changes of HBA1c during 4 months of follow up in the placebo group shows an increase of 0.3% in contrast to the intervention group (Table 3). Comparison at baseline, levels of MDA has not shown any significant difference among two groups (P = 0.67), but at the end of the study trend of decline in MDA concentration in Pioglitazone group was statistically significant whereas in placebo group MDA have not shown such a decline leading to a significant difference in MDA levels between two groups at the end of study (P < 0.0001, 1.22 - 3.90). Regarding the TPC level, the changes were not statistically different at baseline and also at the end of the study between two groups (P = 0.98).
| Pioglitazone Group (N = 31) | Placebo Group (N = 31) | P Values | |
|---|---|---|---|
| Age (years) | 50.2 ± 12.6 | 54.8 ± 8.7 | 0.24 |
| Male sex | 24 (77.4) | 16 (51.6) | 0.06 |
| Weight (kg) | 74.4 ± 14.4 | 76.5 ± 3.7 | 0.68 |
| Time since transplantation (month) | 40 ± 28 | 51 ± 50 | 0.45 |
| Secondary trans. | 1 | 1 | 1.0 |
| Cigarette smoking | 0 | 0 | 1.0 |
| Cadaveric | 10 | 7 | 0.57 |
| Medication Consumption to Treat Underlying Conditions | |||
| Mean insulin NPH dose (IU/day) | 38.2 ± 23.7 | 34.1 ± 14.3 | 0.78 |
| Mean insulin regular dose (IU/day) | 18.0 ± 12.4 | 17.3 ± 14.3 | 0.67 |
| ACEIs | 2 (6.5) | 3 (9.7) | 1.00 |
| ARBs | 13 (41.9) | 10 (32.3) | 0.43 |
| ASA | 11 (35.5) | 15 (48.4) | 0.30 |
| Allopurinol | 6 (19.4) | 5 (16.1) | 0.74 |
| Statins | 19 (61.2) | 17 (54.8) | 0.46 |
| Vitamin E, C | 0 | 0 | 1.00 |
| Prednisolone dose (mg/day) | 6.0 ± 1.9 | 5.9 ± 2.8 | 0.39 |
| Cyclosporine dose (mg/day) | 138.4 ± 33 | 139.8 ± 48.1 | 0.90 |
| Cyclosporine dose (mg/kg/day) | 2.04 ± 0.75 | 1.84 ± 0.74 | 0.23 |
aData are presented as means ± SD or No. (%).
| Pioglitazones Group (N = 31) | Placebo Group (N = 31) | P Values | |
|---|---|---|---|
| FBS (mg/dL) | 136.0 ± 61.6 | 145.5 ± 84.8 | 0.82 |
| HBA1c (%) | 8.6 ± 1.9 | 7.8 ± 1.6 | 0.19 |
| MDA (nmol/mL) | 4.62 ± 2.08 | 4.41 ± 2.08 | 0.67 |
| TPC (nmol/mL) | 1.89 ± 1.03 | 1.75 ± 0.97 | 0.67 |
| Creatinine (mg/dL) | 1.59 ± 0.31 | 1.38 ± 0.45 | 0.34 |
| Blood urea nitrogen (mg/dL) | 30.48 ± 12.41 | 29.97 ± 13.61 | 0.99 |
| Pioglitazone Group (N = 31) | Placebo Group (N = 31) | P Value | |
|---|---|---|---|
| Δ HBA1c (%) | -1.21 ± 1.2 | 0.39 ± 1 | (0.0001, 1.04 - 2.18) |
| Δ MDA (nmol/ml) | - 1.11 ± 1.70 | 1.45 ± 3.32 | (< 0.0001, 1.22 – 3.90) |
| Δ TPC (nmol/ml) | 0.11 ± 1.4 | 0.08 ± 1.52 | (0.98) |
4.1. Safety of Treatment
None of the patients in the intervention group had any experience of severe congestive heart failure and elevation in liver enzymes for 2 or more folds as a serious adverse effect which could be seen with Pioglitazone consumption. Drop out because of adverse drug effects that were seen in 4 patients on the Pioglitazone group. Three of them had lower extremity edema, and one patient experiences insomnia, which led to discontinuation of treatment. All of the other patients tolerated the pioglitazone well and had no trouble with the medication.
5. Discussion
The primary endpoint of the study was looking at changes in MDA and TPC levels and secondarily screening the glycemic control indices, HBA1c. In several studies, it has been shown that oxidative stress plays a fundamental role in the pathogenesis of diabetes consequences (24, 25). As the results of our study showed, the level of MDA was decreased in the pioglitazone group. Previous animal or human reports showed increased levels of MDA indicating the development of oxidative stress in hyperglycemia conditions which were reversed by pioglitazone. For example, Singh et al. Showed that Pioglitazone and metformin significantly reduced MDA in patients with type 2 diabetes mellitus, and pioglitazone was more effective than metformin (26). Also, Wang. et al. showed antioxidant effects of pioglitazone in insulin-resistant diabetic rat models (27). Pioglitazone also could improve long-term serum glucose control and reduce HBA1c as an index for 3 months glycemic control and it could affect inflammatory markers and improve the state of inflammation showed by reducing markers such as erythrocyte sedimentation rate, C-reactive Protein, high-sensitivity CRP, and IL-18. Another study showed that Pioglitazone reduced the level of MDA and increased the level of superoxide dismutase in the damaged kidney tissue (28). An explanation for this anti-oxidative effect is that Pioglitazone can suppress p22phox and p47phox expressions and ultimately reduces oxidative stress in rat mesangial cells under hyperglycemia conditions. p22phox and p47phox are two subunits of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase. This enzyme plays a major role in the production of reactive oxygen species (29). Increasing oxidative stress in diabetic patients can also lead to insulin resistance (6). Pioglitazone improved oxidative stress as reflected by a reduction in MDA (26). this medication exerts its blood glucose-lowering effect by antioxidant activity throw inhibiting the ROS production and inflammatory pathways, as well as increasing insulin sensitivity (30).
In this study, the serum level of TPC was not declined in the Pioglitazone group and also TPC improvement during the 4 months of follow up in the placebo group was not statistically different, when compared two groups from the beginning of the study to the end, there were not any changes lead to a difference. It was contrary to the results of Kavitha et al. study. They showed that the level of protein carbonyls decreased in a group of diabetic rats treated with insulin and Pioglitazone (30). Faruk Turgut confirmed that Pioglitazone could inhibit glycation in vitro (31).
The reaction between the advanced glycation end products (AGEs) and their receptors could be regulated by pioglitazone, by decreasing the expression of the receptor for AGEs on endothelial cells surface and thereafter reducing cell damage and apoptosis (32). One of the complications of chronic hyperglycemia state is the glycosylation of various molecules such as proteins, and the formation of AGEs. Following the increase in AGEs concentration and binding to their receptors (RAGE), the membrane of the glomerular vasculature is thinned and causes fibrosis (33). As declared in previous studies, Pioglitazone is an effective agent for the treatment of diabetes in this population, as evidenced by improvement in HgbA1c levels (34-36). This occurred despite an overall decrease in total daily insulin requirements, which we have shown in our previous study. Total daily dose requirement of NPH Insulin at the end of the study in the Pioglitazone group decreased whereas in placebo group this requirement slightly increased, despite no significant changes in regular insulin total dose in together groups. As Werzowa et al. showed in their study, HBA1c was decreased in both treatment arms which included vildagliptin and pioglitazone and were of potential benefit in patients with IGT after renal transplantation in addition to lifestyle modification (37). There was a significant improvement in the HBA1c level in this group. This can be in part due to Pioglitazone increases glucose uptake by increasing the expression of GLUT4 and IRS-2 (insulin receptor substrate-2) in some tissues (37). There was some limitation to our study, first of all, and probably sources of bias in our study may be due to the limited sample size or the period of observation in this trial. For a decision about the long-term effect of reduction in oxidative state and clinical consequences in diabetic kidney transplanted patients, these effects should be evaluated in long-term surveys. Pioglitazone should be compared with other medications that could affect oxidative stress and pathologic consequences of this state. We concluded that in renal transplant recipients, Pioglitazone not only improves glycemic control (evidenced by lower HBA1c) but also leads to a significant decrement of MDA (a protein oxidative stress marker) in our trial.