Abstract
Background:
After cardiovascular diseases, cancer is the main cause of death in the United States, and its prevalence is continually increasing. Ovarian cancer is a fetal and common cancer among women and is the eighth common cancer in Iran. Colorectal cancer is known as the second and fourth common cancer in Iranian women and men, respectively. Arazyme is a metalloprotease with strong antitumor effects on tumor cells.Objectives:
This study aimed at studying the effect of metalloprotease arazyme in vitro on the expression of MMP2 and MMP9 genes, causing metastasis in ovarian and colon cancer.Methods:
Bacterial strains and cell lines, the construction of an expression vector, and preparation of recombinant protein were done. Then, they were evaluated by Western blot, cell culture, cell viability assay, and reverse transcriptase-polymerase chain reaction.Results:
The effects of arazyme on ovarian and colon cell lines were assessed by the MTT assay showing that the viability of cancer cells treated with arazyme decreased significantly in comparison with control cells. Also, RT-PCR showed that the expression of MMP2 and MMP9 genes decreased after treatment with arazyme, which was significant when compared to the results of pre-treatment.Conclusions:
In this study, the results showed that the use of arazyme protein as a bacterial anti-protease can play a significant role in reducing the expression of metastatic genes. According to numerous studies on the role of bacterial proteases in the process of metastasis in recent years, this method can be considered as a therapeutic approach in reducing the metastatic process.Keywords
1. Background
As one of the leading causes of death in the United States, cancer is dramatically increasing in prevalence. Cancer refers to the uncontrolled growth of cells that results in a condition in which the cell cycle of cancerous cells becomes shorter than that of the same cells in a normal state. When a cell is cancerous, other cells continue their division by receiving minimal natural stimuli (1). Lifestyle changes due to the ever-growing and quick urbanization in advanced countries can cause mortality related to ovarian cancer (2). Ovarian cancer is the eighth common cancer in Iran and is probably the most common gynecologic-related cancer with a survival rate of 615 in five years (3). Colorectal cancer is one of the most common diseases among American and Western European populations that is ranked as the second cause of death in the United States. It is also one of the leading causes of cancer, death and disability globally (4). Colon cancer is ranked third in the list of most common cancers in the United States. Despite all valuable and significant advances in medicine in diagnosis, surgery, and chemotherapy, one-third of patients die from other diseases and metastases (5). Metalloproteinases-2 (MMP-2) and -9 (MMP-9) can control cell apoptosis and proliferation, degrade type IV collagen, and have the potential to destruct the basement membrane (6, 7). Arazyme is a 51.5 kDa metalloprotease of Serratia proteamaculans and is a symbiotic bacterium from the Nephila clavata spider, encoded by the araA gene (8). The metalloprotease arazyme has strong antitumor effects in murine metastatic melanoma by inducing cleavages in the surface of CD44 tumor cells and tumor matrix MMP-8 antibodies. Arazyme increases the activation of markers and secretion of proinflammatory cytokines through TLR4-MyD88-TRIF and MAPK-dependent signaling pathways, along with its proteolytic-dependent activity. It can also increase IFNγ-dependent, CD8+, CD4+, T, and B lymphocyte responses that are known as antitumor responses (9).
2. Objectives
Finding a treatment approach for cancer, as one of the most common diseases in this era, is vital. Based on the above information, arazyme can be used as a medicine to treat cancer patients. Using this enzyme is a potentially beneficial treatment method for many patients and can extend the lifespan of patients. In this study, the effect of this enzyme was investigated on metastatic factor genes, including MMP-2 and MMP-9.
3. Methods
3.1. Bacterial Strains and Cell Lines
Escherichia coli BL21 (DE3) as a bacterial expression host was preserved in our laboratory. The HT-29 (human colorectal adenocarcinoma) and HEK293 (human embryonic kidney 293) cell lines were prepared from the Pasteur Institute, Tehran, Iran.
3.2. Expression Vector Construction
The complete arazyme-encoded gene (araA) of S. proteamaculans (GenBank Accession No. AY818193.1) was implemented in the expression vector pET28a in a container that had a kanamycin-resistant gene, a T7 promoter, and the C-terminal six-His-tagged sequence. The restriction sites BamHI and XhoI (Fermentas, Lithonia) were placed at the 5′ and 3′ ends of the araA gene, respectively. Then, the recombinant gene pET28a/araA was synthesized by Biomatik Corporation (Cambridge, Ont., Canada) and subsequently verified by PCR, after cleaving with BamHI and XHoI.
3.3. Preparation of Recombinant Protein
Overall, after overexpression of the recombinant gene, the protein was purified by a Ni-NTA agarose-based procedure following the on-column re-solubilization protocol. Briefly, the overexpression of r-arazyme was induced by adding 1 mM isopropyl β-D-1-thiogalactopyranoside (IPTG; Sigma, USA) to E. coli BL21 (DE3) carrying the recombinant gene construct culture for 2, 4, 6, and 12 hours. Next, pelleted bacterial cells were dissolved in a lysis buffer (50 mM NaH2PO4, 300 mM NaCl, and 1 mM PMSF with pH 8.0). After the centrifugation of suspension, the supernatant was mixed with Ni-NTA resins (QIAGEN, Germany), and treated with washing buffers with descending urea concentrations (8, 6, 4, 2, 1, and 0 M). Finally, arazyme was eluted with 250 mM imidazole solution. For imidazole removal, the protein solution was dialyzed against Phosphate-buffered Saline (PBS, pH 7.4) for 24 hours. The total quantity of purified and solubilized proteins obtained from one liter of bacterial culture was measured by a NanoDrop 2000 spectrophotometer system (ThermoScientific, USA). In addition, whether the r-arazyme protein was contaminated with Lipopolysaccharide (LPS) or not was examined by a commercial Pierce™ LAL Chromogenic Endotoxin Quantitation Kit.
3.4. Western Blot Assay
After the separation of Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE) containing 12.5% polyacrylamide mini-gels, r-arazyme was transferred onto Polyvinylidene Difluoride (PVDF) membranes (Invitrogen) (10) (Hi-bond Amersham Biosciences, USA) using a semi-dry blotting machine (Trans-Blot®SD, Bio-Rad, USA) at 15 volts/10 min (Labconco, Kansas City, MO, USA). After electrotransferring proteins to a PVDF membrane in a semi-dry transfer cell, the membrane was washed with Tris-buffered Saline Tween 20 (TBST) (Merck, Germany), and blocked with PBS with 1% (w/v) skim milk for one hour. Next, the membrane was incubated with mouse anti-His-tag monoclonal antibody overnight at the temperature of 4°C, followed by 1:10,000 diluted Horseradish Peroxidase (HRP)-conjugated goat anti-mouse antibodies (Sigma, USA). Finally, after washing with TBST (three times each for 5 minutes), the membrane was stained using a 3,3'-Diaminobenzidine (DAB) substrate, and the imaging process was conducted under a digital camera. De-staining was performed by 50% methanol plus 7% acetic acid (5 to 10 minutes at RT).
3.5. Cell Culture
The HT-29, SKOV3 (human ovarian cancer), and HEK293 cell lines were purchased from the Pasteur Institute (Tehran, Iran). Cell lines were cultured as a monolayer in the RPMI-1640 medium (Gibco, Germany) containing 1% penicillin/streptomycin (100 U/mL penicillin and 100 mg/L streptomycin 100 μg/mL; Gibco) and 10% Fetal Bovine Serum (FBS, Gibco) at a temperature of 37°C in the presence of 5% CO2.
3.6. Cell Viability Assay
The MTT assay was performed to determine cell viability. Briefly, 1 × 105 cells/mL were seeded to 96-well plates (Nunc; Naperville, IL) and treated with 5, 10, 16, 32, 64, 128, and 256 µg/mL of r-arazyme for 24 hours. Then, cells were subsequently treated with MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) solution (Sigma-Aldrich; Merck Millipore) at 37°C in a 5% CO2 incubator for 4 hours. Next, the culture medium was replaced by 200 µL of DMSO, and the absorbance was measured at 570 nm using an ELIS Amicro plate reader (11). The percentage of cytotoxicity activity was calculated as follows:
Cytotoxicity activity (%) = [1 - (absorbance of experimental well/ absorbance of negative control well)] × 100
3.7. Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
The mRNA expression of MMP2 and MMP9 was measured by SYBR Green real-time PCR analysis using specific oligonucleotide primers. Then, the total RNA of treated and untreated cells was extracted using a TRIZOL reagent (Invitrogen, USA), which was used for cDNA synthesis by implementing a Revert Aid TM First Strand cDNA Synthesis Kit (Fermentas, Lithonia). The Glyceraldehyde 3-phosphate Dehydrogenase (GAPDH) gene was utilized as an endogenous control. The PCR mixture included SYBR Green Master Mix (12.5 µL), template DNA (400 ng), forward and reverse primers (each 0.25 µM), and nuclease-free water (12 µL). Finally, after a step of denaturation at 95 °C for 5 minutes, RT-PCR was cycled for 40 times between 95°C (for 15 s) and 60 °C (for 60 seconds).
3.8. Statistical Analysis
Quantitative statistical analyses were performed by SPSS version 21 (Armonk, NY: IBM Corp.) and GraphPad Prism 6 (La Jolla, California, USA). Normally distributed data were analyzed by ANOVA, followed by Tukey’s multiple comparison tests. Nonparametric data were analyzed by Kruskal-Wallis ANOVA, followed by multiple comparison tests. All results were considered significant at P ≤ 0.05 and expressed as mean ± SEM.
4. Results
4.1. Final Affirmation of Gene by Enzymatic Digestion Procedure
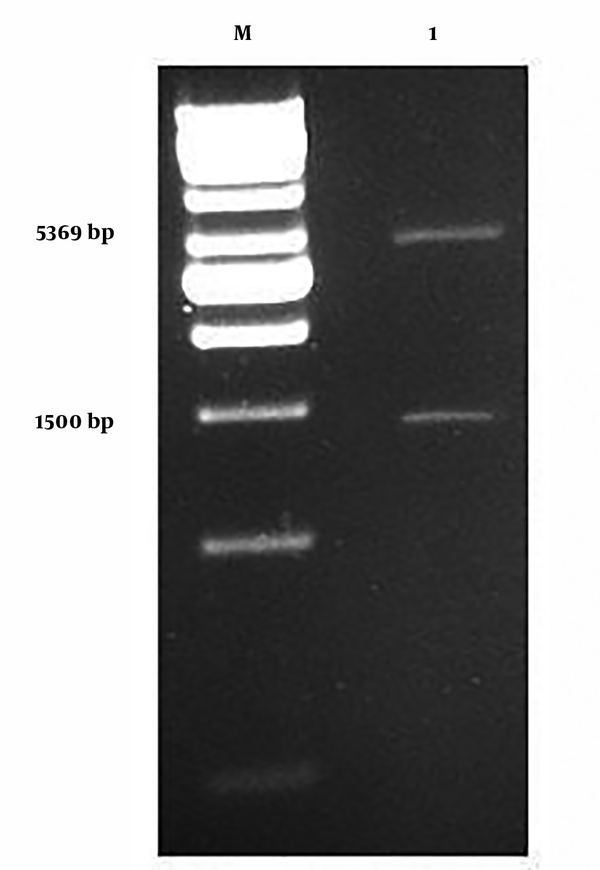
As seen in Figure 1, in two enzymatic digestions with BamHI and XhoI, the araA section was separated from the neo-structure’s vector and placed in its section, which was 1500 bp, while the pET-28a vector did not have a section in 5369 bp.
1, Neo-structure’s vector of digested pET-28a/araA with BamHiand Xhol enzymes and exit of section araA; M, in the shape of DNA marker.
4.2. Expression of Neo-structure’s Arazyme in E. coli
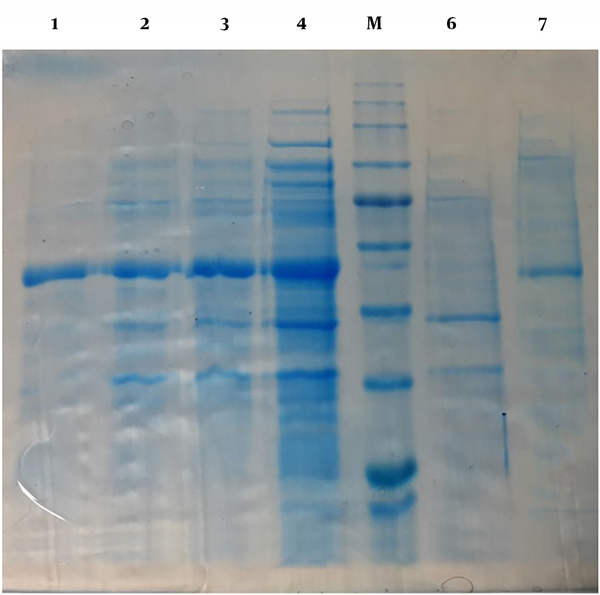
After the transformation of neo-structure’s vector in the expression of BL-21(DE3) and implementation of culture and induction levels, results of SDS PAGE and araA gene expression by IPTG showed by IPTG and production of neo-structure’s Arazyme in 53 KD situations by 5 selected colonies, compared to non-induction situations (Figure 2). Meanwhile, different tests on the culture supernatant and bacterial tests showed that most produced proteins in the shape of windy oncogene were accumulated inside the bacterial cytoplasm and the best conditions that caused high expression levels of the protein were 12 hours of expression with 1mM IPTG at 37˚C.
The expression of neo-structure’s Arazyme protein in E. coli, BL-21(DE3) with SDS-PAGE. 1, Bacterial Lizat resulted from one non-inducted neo-structural colon (control of expression); 2, 3, 4, and 5, Inducted bacterial Lizat with IPTG, 2, 4,6, and 12 hours after induction with IPTG, respectively; 6, Bacterial Lizat resulted from the non-inducted neo-structural colon (control of expression); 7, Reduced Arazyme protein. M; Protein marker.
4.3. Western Blot Test
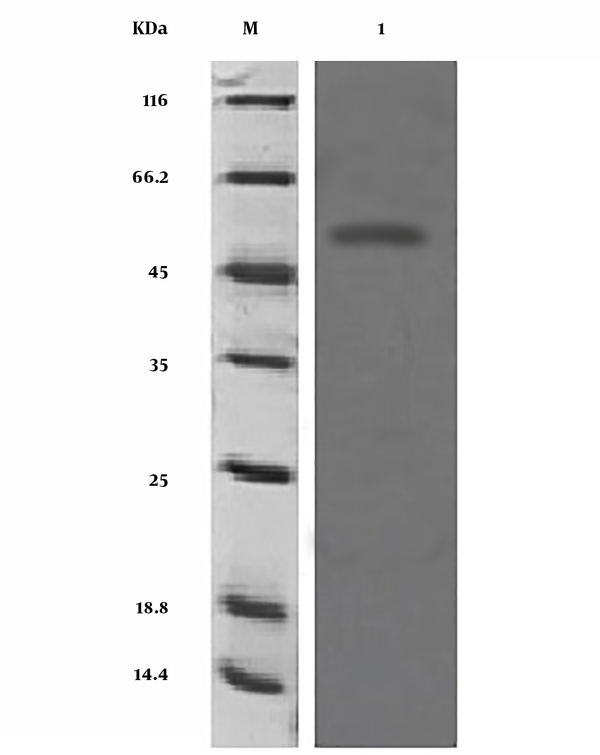
Since the SDS-PAGE procedure did not show protein expression with the specified molecular weight, the Western blot test was applied for affirming the expressed protein. By applying Western blot, it was specified that the anti-polyhistidine monoclonal antibody reacted against the final histidine label in neo-structural arazyme (Figure 3).
Neo-structural proteins of Western blot. 1, Neo-structural proteins of Arazyme; M, Marker.
4.4. MTT Analysis
Tetrazolium yellow salt was used to evaluate the viability of cells treated with arazyme. This salt was absorbed by living cells and formed insoluble Formazan purple crystals. This color was assessed with the spectrometry procedure. For each cell, there was a linear relationship between living cells and the absorption rate, and this relationship provided the possibility to exactly measure the changes in the rates of cell duplication. Figure 4 shows ovarian cancer cells (SKOV3) (A), colon cancer cells (HT29) (B), and HEK293 control cell (C), which were treated with arazyme. The rate of cell duplication was measured under arazyme concentrations of 16 to 256 µg/mL. By comparing the viability rates of cancerous cells treated with arazyme and control cells, the difference in the average percentage of viability was statistically significant at the level of P < 0.05 between treated cells and the control group at different doses (Figure 4A, 4B, and 4C).
The rate of living cells in control and test groups treated with arazyme. The results show a statistically significant difference between the test and control groups (P < 0.05). A; Comparing the control group and ovarian cancer cell. B, Comparing the control group and colon cancer cell; C, Comparing the control group and normal cell HEK293. *, P < 0.05; **, P < 0.01.

4.5. Results of RT-PCR
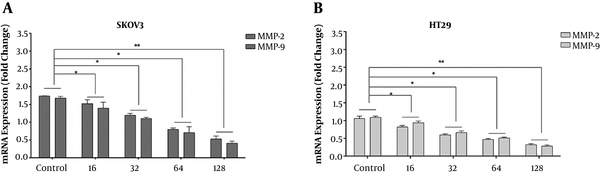
Treating colon and ovarian cancer cells with arazyme caused significant reductions (P < 0.05) in the expression of MMP-2 and MMP-9 metastatic genes when compared to the control group (Figure 5A and 5B).
RT-PCR results of gene expression involved in apoptosis, angiogenesis, and metastasis of ovarian (A) and colon (B) cancer cells treated with Arazyme (P < 0.05).
5. Discussion
Cancer refers to diseases in which abnormal cells uncontrollably divide and obtain the capacity to invade other tissues. Cancerous cells are replicated arbitrarily and continuously. Cancerous cells infiltrate the adjacent normal tissues and interfere with their normal functions. In regulatory systems, this causes irreversible changes and affects normal cell growth and development mechanisms. Changes in the levels of cancer cells' genes are inherited by their next generation. More than 200 types of cancers have been identified in humans so far (12).
Among cancers of the female genital tract, ovarian cancer has been investigated greatly in clinical studies. It has the highest mortality rate among all female genital malignancies and is currently the most common cause of death among all women's cancers worldwide. Various treatment procedures have been studied for the treatment of this disease, each of which is used according to the type of the disease and its conditions. These include chemotherapy, hormone drugs, metastasis prevention drugs, etc. (13). Colorectal cancer, as the second leading cause of cancer death, is caused by a series of histopathologic changes that result from specific genetic alterations in several oncogenes and suppressor tumor genes (14). Despite all efforts made to control metastatic cancers in recent decades, they are still very lethal. New traditional drugs are currently using for chemotherapy in clinics; however, they do not show expected results.
The increasing evidence shows that some natural compounds have the potential for cancer prevention and treatment. Many anticancer agents that are available today are natural products derived from plants or their analogs. However, other new approaches are still needed to overcome the problems of cancer treatment. Recently, bacterial-derived products have been considered as a new strategy in modulating the response to a variety of human diseases, and several compounds have been described as immune-stimulating agents (5, 14, 15). In the present study, malignant cancer cells SKOV3 and HT29 were selected and assessed as models to determine the anti-metastatic effects of arazyme in vitro. Cytoplasmic expression of recombinant protein arazyme was carried out in the form of oncogene particles and by purification. The recombinant protein was collected from the column in high purity. The recombinant protein yield was 4 mg/mL. Due to the high efficiency of the recombinant protein that was largely free of other contaminating proteins, the survival rate of arazyme-treated and untreated cancer cells in the MTT assay indicated a special effect of this protein on reducing cell viability. The study of the efficacy of bacterial proteases on the therapeutic and metastatic processes of cancer has given much attention in recent years. For example, we studied the effect of bacterial serine Htr A protease on lung cancer, which showed to reduce the metastasis process in different ways. Serine Htr A1 protease has also shown to reduce the process of metastasis in melanoma, and protease of Escherichia coli can reduce the process of this disease (15, 16).
The MMP-2 and MMP-9 genes are among the most favorable genes for metastasis screening. A review by Hilary et al. showed the important role of the MMP-2 gene in ovarian cancer. The role of these two genes in gastric cancer indicates that they are very important in the progression of the disease (17). In this study, decreased expression of these two genes with arazyme protease indicated the importance of this protease for the metastasis process after treatment. Treating colon and ovarian cancer cells with arazyme caused significant reductions (P < 0.05) in the expression of MMP-2 and MMP-9 metastatic genes when compared to the control group. Some studies have shown that proteases may have a blocking effect on the tumor. In addition, the administration of exogenous proteases, such as trypsin and chymotrypsin could effectively inhibit tumor growth and development in animal models that were experimentally affected by cancer (18). A study of bacterial-derived metalloprotease arazyme showed that metalloprotease inhibited the development and progression of malignant skin cancer (19). It can be concluded that arazyme could inhibit the MMP-2 and MMP-9 genes, which are related to tumors and metastases. In the present study, performed for the first time on recombinant arazyme, this protease could inhibit metastasis in colorectal and ovarian cancer, similar to previous research, as investigated by RT-PCR of MMP2 and MMP9 genes. The results showed that recombinant arazyme had a high potential to inhibit cell proliferation and death in ovarian and colorectal cancer cells.
5.1. Conclusions
This study showed that arazyme had a little effect on normal cell growth and metabolism and inhibited metastasis in both ovarian and colon cancer cell lines. However, more research should be done on the clinical application of arazyme as a therapeutic agent. By extending this study into the animal phase, we hope excellent results can be achieved and this can be a new combination approach to treat cancer and reduce its mortality.
References
-
1.
McGuire Iii WP, Markman M. Primary ovarian cancer chemotherapy: current standards of care. British journal of cancer. 2003;89(S3). S3.
-
2.
Reid BM, Permuth JB, Sellers TA. Epidemiology of ovarian cancer: a review. Cancer biology & medicine. 2017;14(1):9-32. [PubMed ID: 28443200]. [PubMed Central ID: PMC5365187]. https://doi.org/10.20892/j.issn.2095-3941.2016.0084.
-
3.
Arab M, Khayamzadeh M, Mohit M, Hosseini M, Anbiaee R, Tabatabaeefar M, et al. Survival of ovarian cancer in Iran: 2000-2004. Asian Pacific journal of cancer prevention : APJCP. 2009;10(4):555-8. [PubMed ID: 19827868].
-
4.
Kinzler KW, Vogelstein B. Lessons from hereditary colorectal cancer. Cell. 1996;87(2):159-70.
-
5.
Boring CC, Squires TS, Tong T. Cancer statistics, 1992. CA: A cancer Journal for Clinicians. 1992;42(1):19-38.
-
6.
Gutman M, Fidler IJ. Biology of human colon cancer metastasis. World journal of surgery. 1995;19(2):226-34.
-
7.
Poste G. Pathogenesis of metastatic disease: implications for current therapy and for the development of new therapeutic strategies. Cancer treatment reports. 1986;70(1):183-99.
-
8.
Kwak J, Lee K, Shin DH, Maeng JS, Park DS, Oh HW, et al. Biochemical and genetic characterization of arazyme, an extracellular metalloprotease produced from Serratia proteamaculans HY-3. Journal of microbiology and biotechnology. 2007;17(5):761-8. [PubMed ID: 18051297].
-
9.
Pereira FV, Ferreira-Guimaraes CA, Paschoalin T, Scutti JA, Melo FM, Silva LS, et al. A natural bacterial-derived product, the metalloprotease arazyme, inhibits metastatic murine melanoma by inducing MMP-8 cross-reactive antibodies. PloS one. 2014;9(4). e96141. [PubMed ID: 24788523]. [PubMed Central ID: PMC4005744]. https://doi.org/10.1371/journal.pone.0096141.
-
10.
Taherian A, Fazilati M, Tebyanian H; Moghadam AT. Optimization of purification procedure for horse F(ab´)2 antivenom against Androctonus crassicauda (Scorpion) venom. Trop J Pharm Res. 2018;17(3):409-14. https://doi.org/10.4314/tjpr.v17i3.4.
-
11.
Seifi Kafshgari H, Yazdanian M, Ranjbar R, Tahmasebi E, Mirsaeed S, Tebyanian H, et al. The effect of Citrullus colocynthis extracts on Streptococcus mutans, Candida albicans, normal gingival fibroblast and breast cancer cells. J Biol Res. 2019;92(1). https://doi.org/10.4081/jbr.2019.8201.
-
12.
Spannuth WA, Sood AK, Coleman RL. Angiogenesis as a strategic target for ovarian cancer therapy. Nature Reviews Clinical Oncology. 2008;5(4):194.
-
13.
Li L, Pan Z, Gao K, Zhang W, Luo Y, Yao Z, et al. Impact of post‑operative hormone replacement therapy on life quality and prognosis in patients with ovarian malignancy. Oncology letters. 2012;3(1):244-9.
-
14.
Malpas JS. Chemotherapy in the management of ovarian carcinoma: a review. Journal of the Royal Society of Medicine. 1979;72(5):357-61.
-
15.
Esposito V, Campioni M, De Luca A, Spugnini EP, Baldi F, Cassandro R, et al. Analysis of HtrA1 serine protease expression in human lung cancer. Anticancer research. 2006;26(5A):3455-9.
-
16.
Baldi A, De Luca A, Morini M, Battista T, Felsani A, Baldi F, et al. The HtrA1 serine protease is down-regulated during human melanoma progression and represses growth of metastatic melanoma cells. Oncogene. 2002;21(43):6684.
-
17.
Kenny HA, Kaur S, Coussens LM, Lengyel E. The initial steps of ovarian cancer cell metastasis are mediated by MMP-2 cleavage of vitronectin and fibronectin. The Journal of clinical investigation. 2008;118(4):1367-79.
-
18.
Zheng H, Takahashi H, Murai Y, Cui Z, Nomoto K, Niwa H, et al. Expressions of MMP-2, MMP-9 and VEGF are closely linked to growth, invasion, metastasis and angiogenesis of gastric carcinoma. Anticancer research. 2006;26(5A):3579-83.
-
19.
Pereira FV, Ferreira-Guimarães CA, Paschoalin T, Scutti JA, Melo FM, Silva LS, et al. A natural bacterial-derived product, the metalloprotease arazyme, inhibits metastatic murine melanoma by inducing MMP-8 cross-reactive antibodies. PloS one. 2014;9(4). e96141.