1. Context
The brain is the body’s control center. There are factors that can disturb this vast and widespread system, including infectious disorders, trauma, stroke, tumor, and neurodegenerative diseases. Neurodegeneration diseases are incurable and debilitating disorders that cause progressive loss of cognitive, memory, and motion functions (1). The prevalence of such risk factors has increased in recent years due to various reasons, such as population aging. These diseases, as one of the major health challenges of the modern world, imposed a huge burden on societies (2). Despite great complexity and diversity in the pathophysiology of these chronic diseases, they have some common characteristics, including disturbance in the quality control system of proteins and the accumulation of defective proteins that cause neurodegenerative changes or death of neurons and decline in their function (1, 3). Currently, the available therapeutic options for these diseases only relieve symptoms, and none of them can remarkably reduce or stop the progression of underlying pathology (4). Hence, there is an extremely urgent need to develop more effective therapies, but it can be achieved only by a deep understanding of mechanisms and agents involved in the development of each disease (5).
In these cases, the nervous system should be treated with therapeutic compounds. In this respect, special attention should be paid to the blood-brain barrier (BBB). The BBB is composed of tight junctions (TJs) of cerebral endothelial cells (ECs), choroid plexus epithelial cells, and cells of the arachnoid epithelium. The extreme intensity of TJs (zonulae occludentes) is one of the key features of BBB, which significantly reduces the penetration of polar solutes through the paracellular diffusional pathways between ECs from blood to the extracellular fluid in the brain (6).
The junction complexes between ECs include adherent’s junctions (AJs) and TJs. In AJs, cadherin proteins span the intercellular gap and are linked to cell cytoplasm by alpha, beta, and gamma-catenin proteins. AJs hold the cell together that support the structure of the tissue. AJs are essential for the formation of TJs, and its disorders result in disruption of the barrier (7). TJs contain more proteins that span the intercellular gap, including occludin and Claudine, and junctional adhesion molecules (JAMs) (7, 8).
This barrier prevents the entry and exit of various compounds into the brain. Due to the presence of TJs, instead of large reticulations, between ECs, the BBB considers the medications as foreign molecules (9). Also, as it is known, there is a high resistance between ECs, which is formed by capillary encapsulation by pericytes and astrocytes (10). Only small molecules, such as water, some gases, and soluble compounds in the fat, can pass the BBB through intercellular diffusion (trans). On the other hand, the transfer of large molecules with high electric charge, polarity, and hydrophilicity (such as glucose amino acid and most drugs) depends on specific proteins that use transfer routes (11).
Therefore, delivery and secretion of drugs to the brain is a challenging issue that requires more attention because of the increased prevalence of malignancies in the immune system, such as Alzheimer’s disease (AD) (12), Parkinson’s disease (PD) (13), Huntington’s disease (HD) (14), and HIV encephalitis (15). BBB prevents the entrance of many medications to the CNS through the circulatory system, which is a challenge for the treatment, diagnosis, and prevention of brain disorders. There are several methods for drug delivery to the brain, most of which have many disadvantages such as high risk, high cost, and lack of compatibility.
Recently, different nanoparticles have been used to pass the BBB and transferring various drugs to the brain for therapeutic purposes or imaging on different parts of the brain. Quantum dots (QDs) or carbon dots, a theranostic agent, are one of the various biocompatible and biodegradable nanoparticles that are used. QDs are semiconductor nanocrystals with special optical properties, such as high photochemical stability, extended wavelength, and also a long fluorescent half-life that are widely used in many fields of life sciences (16-18). The advantages and disadvantages of QDs are shown in Table 1.
| Advantages of QDs | Disadvantages of QDs |
|---|---|
| Photostability | CdSe based nanoparticles are highly toxic |
| Wide absorption spectra | and require a stable polymer shell |
| Adjustable wavelengths | The shells can alter the optical properties |
| Ability to measure single or small receptors attached to the ligand | Overall conversion efficiency is lower |
| Detected single or small receptor-ligand- | Lower temperature operation |
| quantum in live cells using for a long time | Controlling the size of these particles is hard |
Some types of conjugated QDs are useful for passing the BBB in various fields, including proper imaging and diagnosis of brain disorders, and are suitable carriers in this regard. The size of these NPs or their chemo-electric charge does not affect the passage of the BBB, while the particle surface is the main factor for transferring the BBB (19).
2. Quantum Dots
One of the important advances in nanotechnology is the synthesis and practical use of QDs, which are semiconductor nanocrystals that contain the atomic elements II-IV or III-V of the Mendeleev and and their size is smaller than Bohr’s exciton for these materials (20, 21). They have photoluminescence controllable properties due to the dimensional quantization effects (22). The importance of QD is due to the significant difference in their exciton discrete spectrum from the bulk crystal spectrum of the same chemical. This difference changes the optical properties of QDs compared to Bulk material. In principle, depending on the chemical structure and size of the QDs, the emission band may be on any sites of the UV light spectrum until infrared. This feature allows receiving marks of various colors for optical coding and use of them for investigating the structures of biological cells.
Advances in QD science and technology came about after 1984 when Luis Brus received the dependence between the size and width of energy for semiconductor nanoparticles (23). However, further studies on the QDs study were needed to progress these nanoparticles. The results of these studies led to the synthesis of colloidal QDs of CdX (X = S, Se, Te) with reconstructed band absorption. So far, CdXs, nanoparticles with extraordinary electrochemical and optical properties, are the most widely studied QDs. However, the use of these particles in biology is impossible because of the toxicity of cadmium ions located at the QD center.
2.1. Graphene Quantum Dots (GQDs)
Graphene is an independent two-dimensional crystal of one atom thickness, one of the hottest topics in materials science, physics, chemistry, and nanotechnology (24). This carbon allotrope consists of layers of six atomic rings in a honeycomb network and conceptually can be represented as a real flat macromolecule (25). As a basic component of other carbon allotropes, graphene can be wrapped to beget 2-dimensional fullerenes, be accelerated to form 1 dimensional carbon nanotubes, and be bunched to produce 3-dimensional graphite (26). Although graphene is the fundamental basis of other carbon forms, it was first set apparently in 2004, 440 years after the invention of graphite, by exfoliation a single layer of graphene using sticky tape and a pencil (27). Several different methods are developed to produce graphene n over the past years. For instance, chemically synthesis, epitaxial growth, chemically pour deposition, and micromechanical blister. Graphene instances produced by the chemical way are also called chemically modified graphene (CMG).
Parallel with the advancement of nanotechnology and biotechnology science, diverse bio/nano interfaces have been found in the areas of biological device design, bioassays, biomolecule detection, and molecular medicine (28-30). Graphene has been used as a substrate to be interfaced with diverse biomolecules and cells. Hence, several studies have focused on the functionalization and modifications of graphene (reviewed in (31)).
3. Neurodegenerative Disorders
3.1. Alzheimer Disease
AD is a progressive neurodegenerative disease and the most prevalent form of dementia among older people worldwide. This irreversible disorder gradually reduces the behavioral and mental functions and the person’s ability to learn (32). The pathophysiology of AD is very complex. One of the most important histopathologic features of AD is the presence of extracellular beta-amyloid (Aβ) plaques and tau protein aggregates in intracellular neurofibrillary tangles (33). More recognized histopathologic features include oxidative stress, mitochondrial dysfunction, neuroinflammation, and BBB dysfunction (34).
Currently, the lack of effective preventive and therapeutic strategies is one of the most serious challenges of patients with AD (35). Acetylcholinesterase inhibitors and glutamate antagonists (N-methyl D-aspartate NMDA) are the most widely used treatments for AD. These pharmacological compounds have a minimal effect on the disease. As a result of obtaining a deeper understanding of the pathophysiology of AD, researchers are more focused on the direct targeting of underlying disease mechanisms (32). New methods for AD treatment are based on inhibiting the production and accumulation of unfolded Aβ proteins and phosphorylated tau and antagonists of the neurotransmitter system. Most of these new therapeutic approaches are highly complex and time-consuming projects.
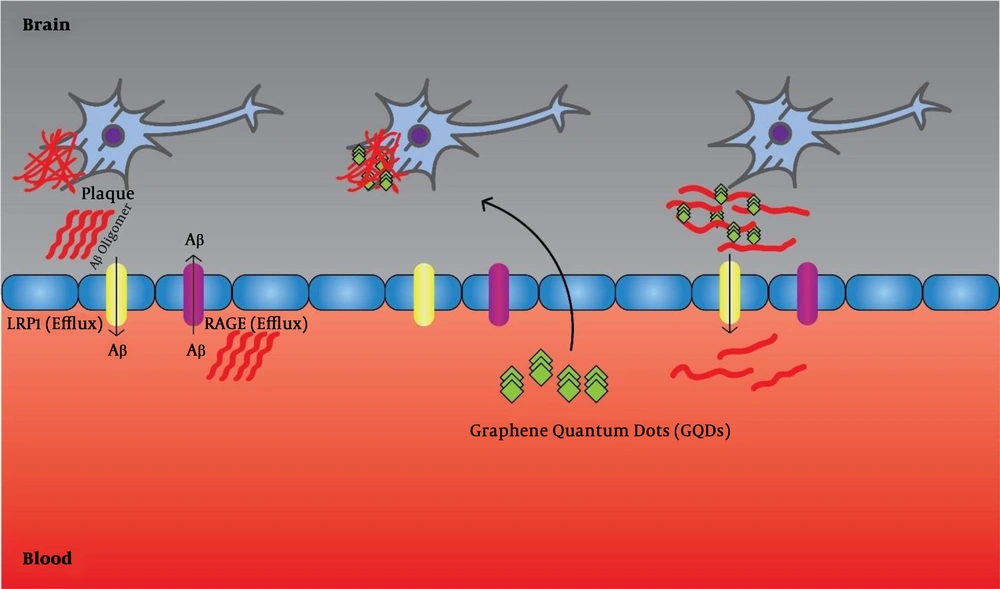
Of course, considering the complex and multifactorial nature of AD, selecting only one molecular target or specific mechanism for its treatment seems impossible. Therefore, new therapeutic approaches should focus on compounds that can target multiple targets (36, 37). Liu et al. (38) showed that GQDs can prevent the accumulation of Aβ1-42 peptides and mentioned therapeutic applications of this complication (Figure 1). Furthermore, Xiao et al. (39), in an in vivo assay using APP/PS1 transgenic mice, used a new nanomaterial combination of GQDs and neuroprotective peptide containing glycine-proline-glutamate (GQDG). They injected this compound into the transgenic mice and reported that it could inhibit the accumulation of AB1-42 fibers, and the level of deposited platelets was lower than the control group. Also, inflammatory cytokines, including TNFα, IL 6, and others, were decreased. Thereby, they found an increase in the memory of mice (39). Tang et al. (40) used a sensitive and reliable sandwich immunoassay to detect the Aβ1-42 peptides using QDs, as fluorescent labels. They showed that under optimal conditions, the linear range of Aβ1-42 assay from 5.0 to 100 pM (0.023 - 0.45 ng mL-1) and the detection limit was reduced to 1.7 pM (7.6 pg.mL-1). Furthermore, they used this method to detect Aβ1-42 in a human cerebrospinal fluid sample and reported successful results (40). Medina-Sanchez et al. (41) evaluated On-chip magneto-immunoassay for AD’s biomarker (apolipoprotein E (ApoE)) electrochemical detection using QDs as labels. They evaluated ApoE detection with high sensitivity and acceptable precision and accuracy. The linear range of ApoE assay ranged from 10 to 200 ng.mL-1, and the detection limit was as low as 12.5 ng.mL-1 and with high accuracy for diluted human plasma (41). Furthermore, Pi et al. (42) developed a sandwich immunoassay for detecting Aβ1-42 using QDs as fluorescent labels. In the presence of Aβ1-42, QDs connected to magnetic beads through the formation of immune-sandwich complex and can be eliminated by a magnetic field. The linear range of Aβ1-42 assay was 0.50 to 8.0nM (2.25 - 36 ng.mL-1) and the detection limit was declined to 0.2 nM (0.9 ng.mL-1) (42).
3.2. Parkinson’s Disease
Second to AD, PD is the most prevalent age-related neurodegenerative disorder (43). The origin of PD is not identified yet. It is estimated that about 2% - 3% of people older than 65 suffer from this disease (44). Despite the mysterious cause of PD, it seems that a series of genetic and environmental factors affect its pathogenesis (45, 46). This progressive disease leads to disturbances in the movement of the patient. The most common pathological feature of PD is the loss of dopaminergic neurons in substantive nigra, followed by the presence of cytoplasmic fibrillary inclusion bodies, which contain ubiquitin and α-syncoline. Other pathogens involved in the mechanism of the disease include apoptosis, mitochondrial dysfunction, oxidative stress, neuroinflammation, and accumulation of misfolded proteins due to ER stress (47). During the past few decades, some treatment approaches, such as drug interventions, deep brain stimulation, and physiotherapy, are developed to relieve the symptoms of PD (48). Pharmacological interventions are based on amplification or replace dopamine in the brain. In addition to dopamine precursor (L-DOPA), some of its agonists, including monoamine oxidase inhibitors and catechol methyltransferase (L-DOPA), are also used in symptomatic treatments for PD. Long-term use of anti-Parkinsonian drugs causes several complications, by altering the levels of neurotransmitters and impairment of hormonal regulation, including various digestive problems, cardiovascular diseases, and mental disorders (49). Also, current therapies relieve symptoms and cannot stop or slow down the process of nerve damage in the brain or improve the patient’s non-motor symptoms. Thus, PD is still an incurable disease. Finding an appropriate therapeutic approach for changing the course of disease progression is one of the greatest challenges to treat PD (46, 49).
PD is a type of brain disorder that is directly linked to the unorganized accumulation of oligomeric and immature alpha-syn in the mid-section of the brain, which causes its toxicity. No inhibitor could successfully treat this disorder yet, but using GQDs, the α-syn accumulation and fibrillation can be prevented. GQDs react directly to adult fibers and dissolve them, reduce mitochondrial disorders, cause loss of synapse, and prevent brain death. In an in vivo study, Kim et al. (50) reported that GQDs could protect Dopamine neurons against matured fibers (50, 51).
In a study intended to control the oligomerization of A53Tαs in the cells, Mohammadi et al. (51) designed a signal-on use of a luciferase split complementation assay and evaluated the effect of GQDs or GOQDs on the oligomerization of A53Tαs. For the first time, they reported that GQDs were at 0.5 µg/mL, which shortened the key phase in fibrillation that is the same nuclear process and can increase the accumulation of A53Tαs and creates a signal on the biosensor. Due to the small size of the GQDs, which causes passing the BBB, the interaction between α-syn and GQDs is useful for PD’s etiology (51). PD is associated with intensified accumulation and transmission of α -synuclein (α-syn) in the midbrain, as a result, no anti-aggregation agent could successfully treat this disease. Kim et al. (50) showed that GQDs could prevent fibrillization of α -syn and interacted straightly with mature fibrils, which triggered their disaggregation. They also observed, in vivo, that GQDs could cross the BBB and protected against dopamine neuron loss induced by α-syn preformed fibrils and behavioral deficits (50). Dopamine (DA) is one of the most important catecholamine neurotransmitters of the human central nervous system that plays several important roles in the brain and body, such as preventing PD and schizophrenia. Daysi et al. synthesized Mn QDs via a facile wet chemical approach for room-temperature detection of dopamine in a vast range of concentrations. They found that the developed sensor displayed high sensitivity and reproducibility to define DA even in biological fluids where DA is at low levels. The sensor exhibited a linear range of ~0.15 - 3.00 μM, and a limit of detection of ~7.80 nM (52).
3.3. Multiple Sclerosis
Multiple sclerosis (MS) is one of the most prevalent neurological diseases and the main cause of nontraumatic neurologic failures in the world. In 2008, the number of people with MS was estimated at 2.1 million, and in 2013, it increased to 2.3 million (53). MS is a chronic inflammatory disorder mediated by T cells that target myelin in the CNS. The pathological characteristics of MS include the presence of separate inflammatory areas, multiple demyelinates plaques, loss of oligodendrocytes, and axonal degradation. Although it seems that MS is an autoimmune disease, its underlying cause is not clear yet (54, 55). There is also evidence suggesting that some environmental factors such as reduced sunlight, vitamin D deficiency, smoking, and some microbial and viral factors, contribute to MS. Mostly young adults and people between the ages of 20 and 50 years are affected by the disease, and its incidence is higher among women than men, by 2 to 3 times (56). MS is thought to be triggered when T cells are activated by the components of myelin in the peripheral immune system and then pass through the BBB and enter the CNS, ultimately causing inflammation and loss of myelin and oligodendrocytes. When inflammation is reduced, remyelination may occur, but the damage to the myelin won’t be restored fully. This can lead to numerous disorders in MS patients’ neurological functions (57, 58). MS patients may present several symptoms such as motor, vision, hearing, and speech problems, sleep disturbances, pain, and acute, chronic fatigue. Depression and mood changes in patients affect their quality of life and social interactions. Despite the significant advances in treatment options for MS over the past two decades, there is still no definitive treatment, only can improve neuronal functions and stop the disease progression (59).
Ten disease-modifying therapies (DMTs) have been approved for the first type of MS (relapsing-remitting), which interferon beta and glutaramasterate are the most important ones. Therapies currently used to treat MS cause several side effects, which most of them are costly to treat. It is estimated that the total annual costs per patient exceed $50,000. Although these methods have been effective in treating some forms of the disease, they failed to prevent or reduce the progression of MS. In addition, they can not fully repair damage to myelin, oligodendrocytes, neurons, and glyca-supporting cells (59, 60).
3.4. Other Neurodegenerative Disease
Huntington’s disease (HD), or Huntington’s chorea, is known as an inherited disorder that results in the destruction of neurons (61). The earliest symptoms are often subtle with problems in mood or mental abilities (62). A general lack of coordination and an unsteady gait often follow (63). With the progression of the disease, jerky uncoordinated body movements become more apparent (1). Subsequently, physical movements gradually worsen until coordinated movement becomes difficult and with difficulties in talking (62, 63). Mental abilities generally cause dementia (64).
Brain-derived neurotrophic factor (BDNF) plays an important role in several facets of neuronal survival, differentiation, and function. Functional and structural aberration in axons are known as an early feature for neurodegenerative diseases, including AD and HD, but the mechanism(s) of axonal injury is not known. Scientists reported a new technique that produces biologically active monobiotinylated BDNF (mBtBDNF) and can be used to trace axonal transport of BDNF. QD-labeled BDNF (QD-BDNF) was produced by conjugating mBtBDNF to QD 655. A microfluidic device can be used to separate axons from neuron cell bodies. The addition of QD-BDNF to the axonal compartment is used to produce live images of BDNF transport in axons. It is also demonstrated that QD-BDNF moved essentially exclusively retrograde, with only a few pauses, at a moving velocity of around 1.06 µm/sec. This system can be used to investigate mechanisms of disrupted axonal function in AD or HD, as well as other degenerative disorders (65).
Prion disease Prions are misfolded proteins that characterize several fatal neurodegenerative diseases both in animals and humans. The cause of misfolding of normal proteins is not clear yet. However, it is believed that an abnormal three-dimensional structure causes infectious properties. The word "Prion" is derived from the "Protein Infectious Particle". The prions are made of prion proteins (PrP), which cause transmissible spongiform encephalopathies (TSEs). Xiao et al. (66) developed a strategy based on two aptamers, recognizing two different epitopes from PrPSc to distinguish it from PrPc in the serum and in brain homogenate. The aptamers are associated with the surface of magnetic microparticles and the QDs. In the presence of PrPSc, it forms a sandwich structure with high fluorescence in the aqueous mediums, which can be separated by an external magnetic field. Xie et al. (67) showed that QD-PEG dispersed in a nitrile acetic acid could be used as a site-specific marker for the PrP expressed at the cell surface in vitro.