Abstract
Keywords
Fibrosis Organophosphate Toxins Physical Activity Poisoning Supplement
1. Background
The population of developing countries is on the rise, leading to increased demand for food products. As agriculture is the primary way of providing the world's food, farmers have no option except to produce food products non-organically. For this purpose, various pesticides are used to control agricultural pests, which in turn translates into increased crop production (1, 2). Organophosphates are agrarian pesticides, and chlorpyrifos is an insecticide of this group. Chlorpyrifos enters the body through the skin, respiratory tract, or gastrointestinal tract and rapidly converts to active metabolites in the liver and kidneys (2, 3).
According to previous studies, heart tissue is one of the organs affected by toxins, resulting in increased apoptosis and disorders (4, 5). Following the heart damage, fibroblasts turn into myofibroblasts that are generally not present in a healthy heart. Myofibroblasts secrete proteins (such as periosteum, collagen I and III, and fibronectin) and are among several cytokines that can regulate the inflammatory response in situ (6). In the case of heart disease, fibrotic regeneration leads to impaired heart function. Myocardial fibroblasts are mechanically and chemically stimulated to differentiate into myofibroblasts in the phenotype (7). TGF-β and Wnt proteins are the two primary regulators of myofibroblasts in cardiac fibrosis (8, 9). Both human and experimental models reported high levels of TGF-β1 expression during cardiac fibrosis (10, 11). In addition, rat models showed increased Wnt / beta-catenin signaling in areas of fibrosis formation (12).
According to the literature, regular exercise and herbs can effectively alter tissue gene expression (13-15). The positive effects of aerobic exercise on improving the impact of chlorpyrifos have been investigated in some studies (16, 17). Eugenol (the active ingredient in cloves) is also highly popular today. Numerous studies mentioned the anti-inflammatory, anti-cancer, anti-apoptotic, antioxidant, and oxidative stress-reducing effects of eugenol supplementation (18-20). Some studies reported reduced harmful effects of chlorpyrifos through eugenol supplementation (16, 17).
While some studies have examined the effect of exercise on Wnt, beta-catenin, and TGF-β (21-23) in cardiac tissue, the effect of exercise on the expression of these genes in chlorpyrifos poisoning has not been studied yet. The effect of eugenol on the expression of Wnt, beta-catenin, and TGF-β gene expression has been investigated by some studies (18, 21-23), but so far, no study has been performed on chlorpyrifos poisoning.
As no study has investigated the expression of these genes in chlorpyrifos poisoning, the present study aimed to investigate the effect of aerobic exercise and eugenol supplementation on the Wnt, beta-catenin, and TGF-β genes expression in the heart tissue of chlorpyrifos-poisoned rats. While each of the research interventions (i.e., practice and complement) may independently affect the expression of the investigated genes, the interaction of two independent interventions may strengthen, modulate, or not differentiate compared to the sole administration of each.
2. Objectives
The present study investigated the interactive effect of aerobic exercise and eugenol supplementation on Wnt, beta-catenin, and TGF-β gene expression in heart tissue.
3. Methods
3.1. Animals
Following an experimental trial design, 42 eight-week-old male Wistar rats weighing 180 to 220 g were obtained from the Pasteur Institute of Iran and randomly divided into seven groups. The animals were transported to the laboratory and kept for one week without any intervention to adapt to the new environment. During this week, rats were randomly divided into seven groups, each with six rats, healthy controls, the healthy receiving DMSO as chlorpyrifos toxin solvent, healthy receiving corn oil as eugenol solvent, poisoned control, aerobic poisoning, eugenol poisoning, and aerobic poisoning eugenol. Rats were kept in transparent polycarbonate cages with dimensions of 42 × 26.5 × 15 cm, temperature of 24°C, 50% humidity, and light cycle in the dark 12: 12 with proper ventilation. The animals were fed daily pellet feed during the study and had free access to the city's tap water through special bottles. All measures performed in this study were designed and implemented based on the instructions for the care and use of laboratory animals in scientific affairs approved by the Ministry of Health and Medical Education of the Islamic Republic of Iran.
3.2. Induction of Chlorpyrifos Poisoning
Chlorpyrifos toxin prepared by Sigma Aldrich Company (USA) was used at a dose of 3 mg/kg for poisoning. Due to the nature of chlorpyrifos toxin, 9% normal saline DMSO solvent was used to dilute the final product. After dissolving, the solution was utterly homogenized by intraperitoneal (IP) injection using a sonicator. The animals received five doses of venom per week for six weeks.
3.3. Aerobic Exercise Program
The exercise used in this study was jogging on rodents. The animals in the training group first worked for two weeks to get acquainted with the daily running method for 20 minutes at a speed of 9 meters per minute. Then, the animals ran on a treadmill for four weeks and five days a week for twenty minutes each day. Running speed started from 16 meters per minute in the first week and increased to 26 meters per minute at the end of the course. Each session contained a warm-up for 5 minutes at a speed of ten meters per minute and a cool down process for 5 minutes at a speed of ten meters per minute. While the duration of the exercise was twenty minutes.
3.4. Eugenol Induction
Eugenol was produced by the Merck Company (German). This substance is a yellow liquid and was used to dilute corn oil solvent based on previous studies. According to the study by Singh et al., the dose was determined as 250 mg/kg and was fed to the rats of the supplement group by gavage for four weeks and five days a week (24).
3.5. Animal Sacrifice and Tissue Resection
The carpet method was used to sacrifice the rats. The histology method was used for cellular and molecular studies. To comply with the ethical standards of rats, blood samples were taken 48 hours after the last intervention with at least 8 hours of fasting with chloroform solution and after splitting the chest from the left ventricle with a three cc syringe. The collected blood was placed in a simple 12 × 100 tubes, and the EDTA tube was placed in a refrigerated centrifuge to collect serum and plasma.
After blood sampling from the heart, the tissues were quickly separated, and the tissue was washed with saline phosphate buffer solution (PBS). Afterward, the tissue was frozen in a nitrogen tank and stored in the freezer's -80° C until analysis. After centrifugation at 3000 rpm at 4° C and 15 minutes, a clear supernatant with a 100 μL sampler for biochemical studies was placed in a 2 mL microtube and transferred to a -80°C freezer until measured.
3.6. Determination of Gene Expression
First, the primer was designed. Total RNA was extracted from the collected cells in each group. The purity of the extracted RNA was evaluated using spectrophotometry and converted to cDNA using reverse transcription enzyme. The resulting cDNA was treated with DNase I to remove genomic DNA and amplified by real-time PCR. The sequence of primers used is presented in Table 1. All kits and instructions were performed according to Rahmati-Ahmadabad et al. (25).
Sequence of Primers Used
| Genes | Forward | Reverse |
|---|---|---|
| WNT | CCTACCTCCCTCCCTCTTCTC | TTCACAAGCTGACCCACCACCA |
| TGF- β | GCCTGGGTTGGAAGTGGAT | GGGTTGTGTTGGTTGTAGAG |
| β-catenin | ATGCTGAGGAAGAAGATGTGGA | ATGAAACTGCGTGGATGGGA |
| rGap | AAGTTCAACGGCACAGTCAAGG | CATACTCAGCACCAGCATCACC |
3.7. Statistical Analysis
Initially, data obtained from gene expression were described using mean and standard deviation. Healthy control groups and toxic control groups were compared using a t-test for independent groups to determine the effect of chlorpyrifos poisoning on the studied outcomes. Then, the control-healthy, toxin solvent, and supplemental groups were compared using a one-way analysis of variance for independent groups to determine the effect of toxin solvent and supplement solvent on the studied outcomes. If a significant difference was observed, the Tukey post hoc test was used to determine the source of the difference. To determine the main effect of exercise, the main effect of eugenol and the interaction of these two interventions were analyzed using a two-way analysis of variance for independent groups. Ben Foroni's post hoc test was also used to determine the location of significant differences. Statistical significance was considered when p-value < 0.05. Data analysis was administered using SPSS version 20.
4. Results
Chloroprifus poisoning significantly increased the expression of WNT, TGF-β, and β-catenin genes (P = 0.001) (Table 2).
The Wnt, TGF-β, and Beta-Catenin Gene Expression in healthy and Chlorpyrifos-Poisoned Control Groups
| Variables | β-catenin | TGF- β | Wnt |
|---|---|---|---|
| Healthy control | 0.0004 ± 0.0001 | 0.023 ± 0.0037 | 0.00081 ± 0.00012 |
| Toxic control a | 0.0008 ± 0.0001 | 0.0559 ± 0.0078 | 0.0021 ± 0.0006 |
Toxin solvent (DMSO) and eugenol solvent (corn oil) had no significant effect on the expression of WNT (T = 0.052), TGF-β (P = 0.598), and beta-catenin (P = 0.219) genes (Table 3).
The Wnt, TGF-β, and Beta-Catenin Gene Expression in Healthy Control Groups, Toxin Solvent (DMSO), and Eugenol Solvent (Corn Oil)
| Variables | β-Catenin | TGF- β | Wnt |
|---|---|---|---|
| Healthy control | 0.0004 ± 0.0001 | 0.023 ± 0.0037 | 0.00081 ± 0.00012 |
| Toxin solvent (DMSO) | 0.0004 ± 0.0001 | 0.022 ± 0.0036 | 0.00083 ± 0.00011 |
| (Corn oil) eugenol solvent | 0.0002 ± 0.00008 | 0.020 ± 0.0081 | 0.00064 ± 0.00013 |
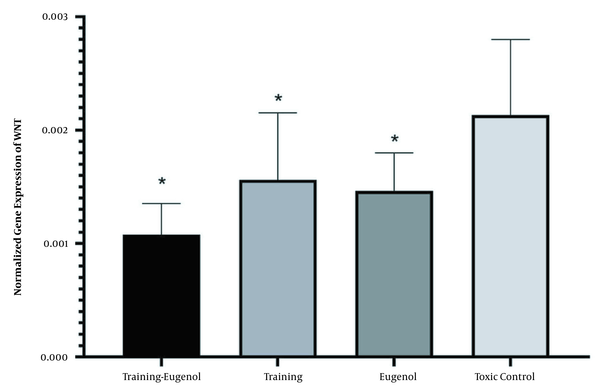
Aerobic exercise significantly reduced the expression of Wnt gene in the heart tissue (F = 5.63, P = 0.028, η = 0.220). Eugenol intake could also significantly reduce the Wnt gene expression in the heart tissue (F = 8.13, P = 0.010, η = 0.289). While the lowest level of Wnt gene expression was observed in the aerobic exercise group and eugenol intake, the interaction of this intervention on Wnt gene expression was not statistically significant (F = 0.21, P = 0.650, η = 0.011). There was no significant difference between the eugenol aerobic exercise and aerobic exercise groups (P = 0.354) and the eugenol group (P = 0.549) concerning Wnt gene expression. There was also no difference between aerobic exercise and eugenol (P = 0.986) (Figure 1).
Expression of Wnt gene of heart tissue in the studied groups (* a significant decrease compared to the control-poisoned group).
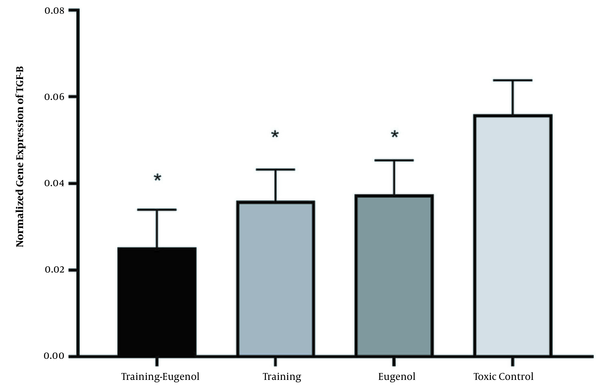
Aerobic exercise decreased the TGF-β gene expression in the heart tissue (F = 24.81, P = 0.001, η = 0.554). Eugenol intake also significantly reduced TGF-β gene expression in heart tissue (F = 20.39, P = 0.001, η = 0.505). While the highest level of TGF-β gene expression was observed in aerobic exercise and eugenol intake groups, the interaction of these two interventions on the TGF-β gene expression was not statistically significant (F = 1.47, P = 0.239, 69 = 0.069). There was no significant difference between the exercise-eugenol group and the exercise (P = 0.123) and the eugenol groups (P = 0.066) concerning the expression of the TGF-β gene. Also, no significant difference in TGF-β gene expression was observed between the training group and eugenol (P = 0.987) (Figure 2).
The TGF-β gene expression in heart tissue in the studied groups (* a significant decrease compared to the control-poisoned group. Information are reported based on mean and standard deviation).
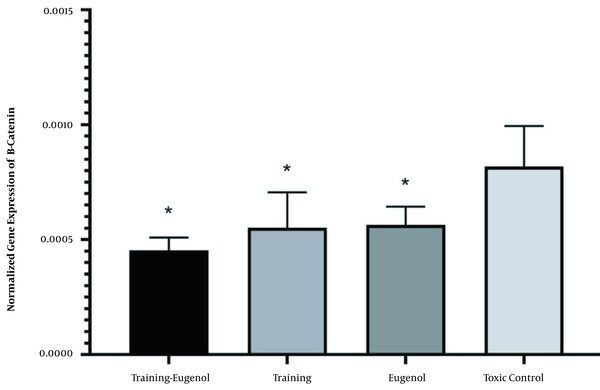
Aerobic exercise significantly reduced the beta-catenin gene expression in the heart tissue (F = 13.15, P = 0.002, η = 0.397). Eugenol intake also significantly reduced the beta-catenin gene expression in the heart tissue (F = 11.63, P = 0.003, η = 0.368). While the lowest level of gene expression of the beta-catenin was observed in the aerobic exercise and eugenol intake groups, the interaction of this intervention on the beta-catenin gene expression was not statistically significant (F = 2.30, P = 0.145, η = 0.103). There was no significant difference between the exercise-eugenol group and the exercise group (P = 0.550) and the eugenol group (P = 0.461) concerning the expression of the beta-catenin gene. There was no significant difference in beta-catenin gene expression between the exercise and eugenol groups (P = 0.999) (Figure 3).
The beta-catenin gene expression in the studied groups (* a significant decrease compared to the control-poisoned group. Information is reported based on mean and standard deviation).
5. Discussion
This study demonstrated that toxicity increases expression of the Wnt, TGFβ, and beta-catenin genes in the heart of rats. The use of each of the exercise and supplement interventions can moderate this reduction. While the expression of these genes in the exercise-eugenol group was significantly lower than that of the toxic control group, its amount in the interactive group was not different from the aerobic exercise and eugenol groups. The combination of exercise and eugenol had no interactive or synergistic effect on the Wnt, TGFβ, and beta-catenin gene expression in the heart of rats poisoned with chlorpyrifos.
Chlorpyrifos is a toxin that affects all tissues. Regarding the toxicity of chlorpyrifos on pancreatic tissue, chlorpyrifos has been shown to interfere with hormonal signaling and metabolism (26). Another study intended to evaluate DNA damage and cytotoxicity in insecticide-poisoned rats reported that acute and chronic insecticide administration caused significant DNA damage in the liver, kidney, brain, and spleen tissues (27).
Regarding the cardiovascular effects of chlorpyrifos, it has been shown that exposure to this substance affects blood pressure and resting heart rate (28). A study on mice exposed to the toxin showed that chlorpyrifos interfered with various parts of the normal heart electrocardiogram (such as an increase in the ST segment), increased blood pressure, and enhanced enzymes in markers of heart tissue damage (such as creatine). Kinase and troponin resulted in decreased cardiac antioxidants (such as superoxide dismutase), increased lipid peroxidation, and enhanced cardiac tissue apoptosis (29). Another study showed that exposure to chlorpyrifos caused heart failure in rabbits (30). Chlorpyrifos induces the production of free radicals and dose-dependent oxidative stress. The most severe adverse effect of chlorpyrifos poisoning is on the liver, which leads to an increase in liver and oxidative enzymes, including aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, catalase, and superoxide dismutase (31). Studies showed that chlorpyrifos at doses of 15 and 30 mg per kg of body weight significantly affected the expression of caspase 3 and 9 genes, BAX, and BCL2 (32). This toxic substance contains organic phosphorus compounds that can react with cell macromolecules and macromolecules and cause cellular and genetic damage. Some researchers suggested increasing lipid peroxidation and production of free radicals resulting from the metabolism of organic phosphorus toxins as the primary mechanism of destruction of cells and various body tissues (31).
Cardiac fibrosis is characterized by an increase in the activity of cardiac fibroblasts, which increases the rigidity of the heart and the risk of heart failure and sudden cardiac death. The TGF-β and WNT signaling pathways are the two central regulators of myofibroblasts in cardiac fibrosis (8, 9). TGF-β1 increases during cardiac fibrosis (10, 11). Wnt/beta-catenin signaling has also been shown to increase in areas of fibrosis formation (12). It seems that aerobic exercise and eugenol supplementation provided in the present study could moderate the expression of these genes, which contribute to heart disease. Some studies have investigated Wnt, beta-catenin, and TGF-β (21-23) in cardiac tissue. In a review study, Tao et al. emphasized the positive role of physical activity in reducing TGF-β (23). Benito et al. showed that intense, long-term physical activity could improve cardiac function by acting on TGF-β (21). Martherus et al. showed that endurance activity improves heart function by regulating the Wnt/beta-catenin signaling pathway (22). The effect of exercise on the expression of these genes in chlorpyrifos poisoning has not been studied yet. The present study showed that exercise and supplementation of eugenol in chlorpyrifos intoxication could modulate the expression of Wnt, beta-catenin, and TGF-β genes.
While the effect of eugenol on the Wnt, beta-catenin, and TGF-β gene expression has been investigated by several studies (18, 21-23), no study has been performed on chlorpyrifos poisoning. Al-Sharif et al. showed the positive role of eugenol in improving breast cancer by regulating TGF-β expression (18). Eugenol has been shown to improve lung cancer by regulating beta-catenin (33). There are many mechanisms associated with the positive effects of aerobic exercise and eugenol. Aerobic exercise removes toxins by increasing blood flow and, consequently, increasing the excretion of toxins from the body. In addition, such effects can be attributed to increased intestinal stimulation for defecation, enhanced antioxidant defense and DNA repair, increased angiogenesis and neurogenesis, reduced free radicals, and increased number and size of mitochondria (34-36). Aerobic activity reduces oxidative stress and DNA damage in rats poisoned with H2O2 (37, 38). It has been shown that aerobic activity can reduce the damage caused by chlorpyrifos toxin (16, 17, 39). Numerous studies have observed the anti-inflammatory, anti-cancer, anti-apoptotic, antioxidant, and oxidative stress-reducing effects of eugenol supplementation (18-20). Some studies found reduced negative effects of chlorpyrifos following eugenol supplementation (18, 24). This study demonstrated the positive role of exercise and eugenol in reducing chronic cardiac toxicity through modifying the expression of Wnt, TGFβ, and beta-catenin genes.
5.1. Practical Achievement
The results of this study can be considered as a basis for future human studies.
Acknowledgements
References
-
1.
Joko T, Dewanti NAY, Dangiran HL. Pesticide Poisoning and the Use of Personal Protective Equipment (PPE) in Indonesian Farmers. J Environ Public Health. 2020;2020:5379619. [PubMed ID: 32405302]. [PubMed Central ID: PMC7201457]. https://doi.org/10.1155/2020/5379619.
-
2.
Sidhu GK, Singh S, Kumar V, Dhanjal DS, Datta S, Singh J. Toxicity, monitoring and biodegradation of organophosphate pesticides: A review. Crit Rev Environ Sci Technol. 2019;49(13):1135-87. https://doi.org/10.1080/10643389.2019.1565554.
-
3.
Gonzalez-Alzaga B, Romero-Molina D, Lopez-Flores I, Gimenez-Asensio MJ, Hernandez AF, Lacasana M. Urinary levels of organophosphate pesticides and predictors of exposure in pre-school and school children living in agricultural and urban communities from south Spain. Environ Res. 2020;186:109459. [PubMed ID: 32335427]. https://doi.org/10.1016/j.envres.2020.109459.
-
4.
Reddy BS, Skaria TG, Polepalli S, Vidyasagar S, Rao M, Kunhikatta V, et al. Factors associated with outcomes in organophosphate and carbamate poisoning: a retrospective study. Toxicol Res. 2020;36(3):257-66. [PubMed ID: 32685430]. [PubMed Central ID: PMC7351927]. https://doi.org/10.1007/s43188-019-00029-x.
-
5.
Roth A, Zellinger I, Arad M, Atsmon J. Organophosphates and the heart. Chest. 1993;103(2):576-82. [PubMed ID: 8432156]. https://doi.org/10.1378/chest.103.2.576.
-
6.
Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3(5):349-63. [PubMed ID: 11988769]. https://doi.org/10.1038/nrm809.
-
7.
Santiago JJ, Dangerfield AL, Rattan SG, Bathe KL, Cunnington RH, Raizman JE, et al. Cardiac fibroblast to myofibroblast differentiation in vivo and in vitro: expression of focal adhesion components in neonatal and adult rat ventricular myofibroblasts. Dev Dyn. 2010;239(6):1573-84. [PubMed ID: 20503355]. https://doi.org/10.1002/dvdy.22280.
-
8.
Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149(6):1192-205. [PubMed ID: 22682243]. https://doi.org/10.1016/j.cell.2012.05.012.
-
9.
Leask A, Abraham DJ. TGF-beta signaling and the fibrotic response. FASEB J. 2004;18(7):816-27. [PubMed ID: 15117886]. https://doi.org/10.1096/fj.03-1273rev.
-
10.
Kuwahara F, Kai H, Tokuda K, Kai M, Takeshita A, Egashira K, et al. Transforming growth factor-beta function blocking prevents myocardial fibrosis and diastolic dysfunction in pressure-overloaded rats. Circulation. 2002;106(1):130-5. [PubMed ID: 12093782]. https://doi.org/10.1161/01.cir.0000020689.12472.e0.
-
11.
Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113(6):685-700. [PubMed ID: 12809600]. https://doi.org/10.1016/s0092-8674(03)00432-x.
-
12.
Blyszczuk P, Muller-Edenborn B, Valenta T, Osto E, Stellato M, Behnke S, et al. Transforming growth factor-beta-dependent Wnt secretion controls myofibroblast formation and myocardial fibrosis progression in experimental autoimmune myocarditis. Eur Heart J. 2017;38(18):1413-25. [PubMed ID: 27099262]. https://doi.org/10.1093/eurheartj/ehw116.
-
13.
Ghanbari-Niaki A, Rahmati-Ahmadabad S. Effects of a fixed-intensity of endurance training and pistacia atlantica supplementation on ATP-binding cassette G4 expression. Chin Med. 2013;8(1):23. [PubMed ID: 24267473]. [PubMed Central ID: PMC4175503]. https://doi.org/10.1186/1749-8546-8-23.
-
14.
Rahmati-Ahmadabad S, Azarbayjani MA, Broom DR, Nasehi M. Effects of high-intensity interval training and flaxseed oil supplement on learning, memory and immobility: relationship with BDNF and TrkB genes. Comp Exerc Physiol. 2021;17(3):273-83. https://doi.org/10.3920/cep200046.
-
15.
Zarezadehmehrizi A, Hong J, Lee J, Rajabi H, Gharakhanlu R, Naghdi N, et al. Exercise training ameliorates cognitive dysfunction in amyloid beta-injected rat model: possible mechanisms of Angiostatin/VEGF signaling. Metab Brain Dis. 2021;36(8):2263-71. [PubMed ID: 34003412]. https://doi.org/10.1007/s11011-021-00751-2.
-
16.
Nikbin S, Derakhshideh A, Hozouri Tarighe M, Khojasteh Z, Kanozi F, Mousavi N, et al. Synergic effects of aerobic exercise and eugenol supplement on germ cell development and testicular tissue structure in chlorpyrifos-treated animal model. Environ Sci Pollut Res Int. 2020;27(14):17229-42. [PubMed ID: 32152857]. https://doi.org/10.1007/s11356-020-08222-4.
-
17.
Nikbin S, Tajik A, Allahyari P, Matin G, Hoseini Roote SS, Barati E, et al. Aerobic exercise and eugenol supplementation ameliorated liver injury induced by chlorpyrifos via modulation acetylcholinesterase activation and antioxidant defense. Environ Toxicol. 2020;35(7):783-93. [PubMed ID: 32096903]. https://doi.org/10.1002/tox.22913.
-
18.
Al-Sharif I, Remmal A, Aboussekhra A. Eugenol triggers apoptosis in breast cancer cells through E2F1/survivin down-regulation. BMC Cancer. 2013;13:600. [PubMed ID: 24330704]. [PubMed Central ID: PMC3931838]. https://doi.org/10.1186/1471-2407-13-600.
-
19.
Jaganathan SK, Mazumdar A, Mondhe D, Mandal M. Apoptotic effect of eugenol in human colon cancer cell lines. Cell Biol Int. 2011;35(6):607-15. [PubMed ID: 21044050]. https://doi.org/10.1042/CBI20100118.
-
20.
Junior PL, Camara DA, Costa AS, Ruiz JL, Levy D, Azevedo RA, et al. Apoptotic effect of eugenol envolves G2/M phase abrogation accompanied by mitochondrial damage and clastogenic effect on cancer cell in vitro. Phytomedicine. 2016;23(7):725-35. [PubMed ID: 27235711]. https://doi.org/10.1016/j.phymed.2016.03.014.
-
21.
Benito B, Gay-Jordi G, Serrano-Mollar A, Guasch E, Shi Y, Tardif JC, et al. Cardiac arrhythmogenic remodeling in a rat model of long-term intensive exercise training. Circulation. 2011;123(1):13-22. [PubMed ID: 21173356]. https://doi.org/10.1161/CIRCULATIONAHA.110.938282.
-
22.
Martherus R, Jain R, Takagi K, Mendsaikhan U, Turdi S, Osinska H, et al. Accelerated cardiac remodeling in desmoplakin transgenic mice in response to endurance exercise is associated with perturbed Wnt/beta-catenin signaling. Am J Physiol Heart Circ Physiol. 2016;310(2):H174-87. [PubMed ID: 26545710]. [PubMed Central ID: PMC4796627]. https://doi.org/10.1152/ajpheart.00295.2015.
-
23.
Tao L, Bei Y, Zhang H, Xiao J, Li X. Exercise for the heart: signaling pathways. Oncotarget. 2015;6(25):20773-84. [PubMed ID: 26318584]. [PubMed Central ID: PMC4673228]. https://doi.org/10.18632/oncotarget.4770.
-
24.
Singh P, Jayaramaiah RH, Agawane SB, Vannuruswamy G, Korwar AM, Anand A, et al. Potential Dual Role of Eugenol in Inhibiting Advanced Glycation End Products in Diabetes: Proteomic and Mechanistic Insights. Sci Rep. 2016;6:18798. [PubMed ID: 26739611]. [PubMed Central ID: PMC4704049]. https://doi.org/10.1038/srep18798.
-
25.
Rahmati-Ahmadabad S, Azarbayjani MA, Farzanegi P, Moradi L. High-intensity interval training has a greater effect on reverse cholesterol transport elements compared with moderate-intensity continuous training in obese male rats. Eur J Prev Cardiol. 2019. [PubMed ID: 33611472]. https://doi.org/10.1177/2047487319887828.
-
26.
Fang B, Li JW, Zhang M, Ren FZ, Pang GF. Chronic chlorpyrifos exposure elicits diet-specific effects on metabolism and the gut microbiome in rats. Food Chem Toxicol. 2018;111:144-52. [PubMed ID: 29109040]. https://doi.org/10.1016/j.fct.2017.11.001.
-
27.
Ojha A, Yaduvanshi SK, Srivastava N. Effect of combined exposure of commonly used organophosphate pesticides on lipid peroxidation and antioxidant enzymes in rat tissues. Pestic Biochem Phys. 2011;99(2):148-56. https://doi.org/10.1016/j.pestbp.2010.11.011.
-
28.
Smith EG, Gordon CJ. The effects of chlorpyrifos on blood pressure and temperature regulation in spontaneously hypertensive rats. Basic Clin Pharmacol Toxicol. 2005;96(6):503-11. [PubMed ID: 15910416]. https://doi.org/10.1111/j.1742-7843.2005.pto_15.x.
-
29.
Wakf AM, M El Habibi ES, Barakat NM, Attia AM, Hussein AM, Ali II. Cardiovascular Toxic Effects of Chlorpyrifos: A Possible Protective Role for Pomegranate Extracts. J Clin Toxicol. 2018;8(1):1000374. https://doi.org/10.4172/2161-0495.1000374.
-
30.
Cetin N, Cetin E, Eraslan G, Bilgili A. Chlorpyrifos induces cardiac dysfunction in rabbits. Res Vet Sci. 2007;82(3):405-8. [PubMed ID: 17064743]. https://doi.org/10.1016/j.rvsc.2006.08.002.
-
31.
Tuzmen N, Candan N, Kaya E, Demiryas N. Biochemical effects of chlorpyrifos and deltamethrin on altered antioxidative defense mechanisms and lipid peroxidation in rat liver. Cell Biochem Funct. 2008;26(1):119-24. [PubMed ID: 17437321]. https://doi.org/10.1002/cbf.1411.
-
32.
Zhang Y, Chang Y, Cao H, Xu W, Li Z, Tao L. Potential threat of Chlorpyrifos to human liver cells via the caspase-dependent mitochondrial pathways. Food Agric Immunol. 2017;29(1):294-305. https://doi.org/10.1080/09540105.2017.1373271.
-
33.
Choudhury P, Barua A, Roy A, Pattanayak R, Bhattacharyya M, Saha P. Eugenol emerges as an elixir by targeting beta-catenin, the central cancer stem cell regulator in lung carcinogenesis: an in vivo and in vitro rationale. Food Funct. 2021;12(3):1063-78. [PubMed ID: 33443517]. https://doi.org/10.1039/d0fo02105a.
-
34.
Bersaoui M, Baldew SM, Cornelis N, Toelsie J, Cornelissen VA. The effect of exercise training on blood pressure in African and Asian populations: A systematic review and meta-analysis of randomized controlled trials. Eur J Prev Cardiol. 2020;27(5):457-72. [PubMed ID: 31450966]. https://doi.org/10.1177/2047487319871233.
-
35.
Herring MP, Puetz TW, O'Connor PJ, Dishman RK. Effect of exercise training on depressive symptoms among patients with a chronic illness: a systematic review and meta-analysis of randomized controlled trials. Arch Intern Med. 2012;172(2):101-11. [PubMed ID: 22271118]. https://doi.org/10.1001/archinternmed.2011.696.
-
36.
Luk TH, Dai YL, Siu CW, Yiu KH, Chan HT, Lee SW, et al. Effect of exercise training on vascular endothelial function in patients with stable coronary artery disease: a randomized controlled trial. Eur J Prev Cardiol. 2012;19(4):830-9. [PubMed ID: 21724681]. https://doi.org/10.1177/1741826711415679.
-
37.
Bahram Vash Shams S, Farzanegi P, Azarbayjany MA. Effects of Aerobic Exercise and Ethanolic Extract of Purslane Seed on Markers of Oxidative Stress and DNA Damage in Cardiac Tissue of Rats Poisoned with Hydrogen Peroxide. Med Lab J. 2021;15(3):40-6.
-
38.
Pirooz M, Azarbayjani MA, Peeri M, Hosseini SA. The Effect of Aerobic Training with Vitamin D on Extrinsic Pathway of Apoptosis and Anti-Apoptotic Indices of Heart Tissue of Rats Exposed to Oxidative Damage Induced by H2O2. Report of Health Care. 2017;3(1):30-40.
-
39.
Soltani-Moez F, Rahmati-Ahmadabad S. The Independent and Combined Effects of Aerobic Exercise Training and Eugenol Consumption on Cardiac Acetylcholinesterase (AChE) in Chlorpyrifos Poisoned Rats. Thrita. 2021;9(2). e112271. https://doi.org/10.5812/thrita.112271.