Abstract
Background:
Controlling parenchymal hemorrhage especially in liver parenchyma, despite the progress in surgical science, is still one of the challenges that surgeons face when trying to save patients’ lives. Researchers in the field are challenging to introduce a more effective method.Objectives:
The current study aimed to determine the haemostatic effect of alum [KAl (SO4)2] and compare it with that of standard method, suturing technique, in controlling bleeding from liver parenchymal tissue.Materials and Methods:
In this animal model study, 60 male Wistar rats were used. An incision, two centimeters long and half a centimeter deep, was made on each rat’s liver and the hemostasis time was measured once using alum with different concentrations (5%, 10%, 15%, 25%, and 50%) and then the control method, controlling bleeding by suturing, was employed. The liver tissue was examined for pathological changes.Results:
Complete hemostasis occurred in all the groups. There was a statistically significant difference between the two haemostatic times (P < 0.001). The haemostatic times in different concentrations of alum were significantly less than that of the control group (P < 0.001). The pathologic examination showed the highest frequency of low-grade inflammation based on the defined pathological grading.Conclusions:
Alum might be an effective haemostatic agent in controlling liver parenchymal tissue hemorrhage in an animal model.Keywords
1. Background
The control of a solid organ’s hemorrhage like liver, due to its rich vascular network, is a challenging task, even in the operation room. The main problem in the hemostasis of liver is the sinusoidal structures of this organ, where the blood vessels are too small to be closed by the routine surgery techniques (1). On the other hand, the number of operations in which liver needs to be cut, such as metastasectomy and liver trauma, is increasing every day (2, 3). The high morbidity and mortality rates of liver injuries are attributed to the too large volume of blood lost and extensive control of bleeding imposed to the patient (4). This has motivated many studies and led to the introduction of new techniques like intermittent clamping of the portal triad to control liver bleeding (5). These studies aimed to introduce a treatment method for liver bleeding control to prevent the complete resection of the bleeding part of the liver (6-9).
Alum is a white chemical agent with formula KAl(SO4)2 and acidic property. Alum exerts its haemostatic effect through a chemical reaction with blood proteins, a property which makes it a very efficient haemostatic agent, without the need for normal body haemostatic system to exert its effect even in patients with abnormal body haemostatic system. It adequately satisfies surgeons’ need to control bleeding (10-12). This property thereof, with regard to the significant amount of proteins in blood, makes alum a very strong haemostatic agent. Although the haemostatic effect of this agent has already been found (11), this effect of alum in controlling liver bleeding and comparing it with the standard method, suturing technique, was not evaluated before.
2. Objectives
The current study aimed to determine the haemostatic effect of alum and compare it with that of standard method, suturing technique, to control bleeding from liver parenchyma.
3. Materials and Methods
3.1. Study Setting and Animals
The current study was conducted at Kashan University of Medical Sciences, Kashan, Iran, from December 2013 to March 2014. In this study, 60 male Wistar rats weighting 180 to 230 g were randomly divided into six groups, each of which included 10 rats. One week before the study, animals were kept at 21 ± 1ºC with 12 hours light/dark cycle, lights on from 8: 00 a.m. to 8: 00 p.m. They had free access to standard rat chaw and water ad libitum. Animal handling and all experiments were performed in accordance with the international guidelines for the care and use of laboratory animals (Institute of Laboratory Animal Resources, 1996) and approved by the local research council at Kashan University of Medical Sciences, Kashan, Iran.
3.2. Surgery
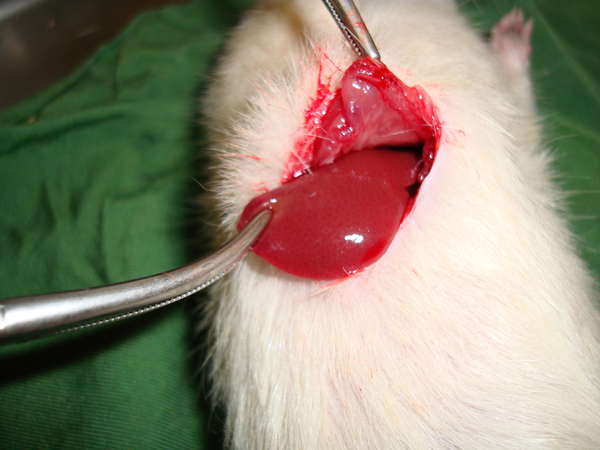
Rats were anesthetized by intraperitoneal (IP) injection of a ketamine/xylazine mixture (ketamine 100 mg/kg and xylazine 10 mg/kg). The 10% ketamine and 2% xylazine (Alfasan, the Netherlands) were purchased from pharmacy at Shahid Beheshti Hospital of Kashan. Then the cutaneous and subcutaneous layers in the abdominal zone were opened, and after determining the anatomical position of the liver, the liver lobe was extracted from the abdominal cavity (Figure 1). Next, a two-centimeter long and half-a-centimeter deep cut was made on the liver by a scalpel, the depth was determined by a mark made on the scalpel 0.5 cm from the tip, and the length was measured with a ruler on the liver.
3.3. Alum Administration and Haemostatic Time Measurement
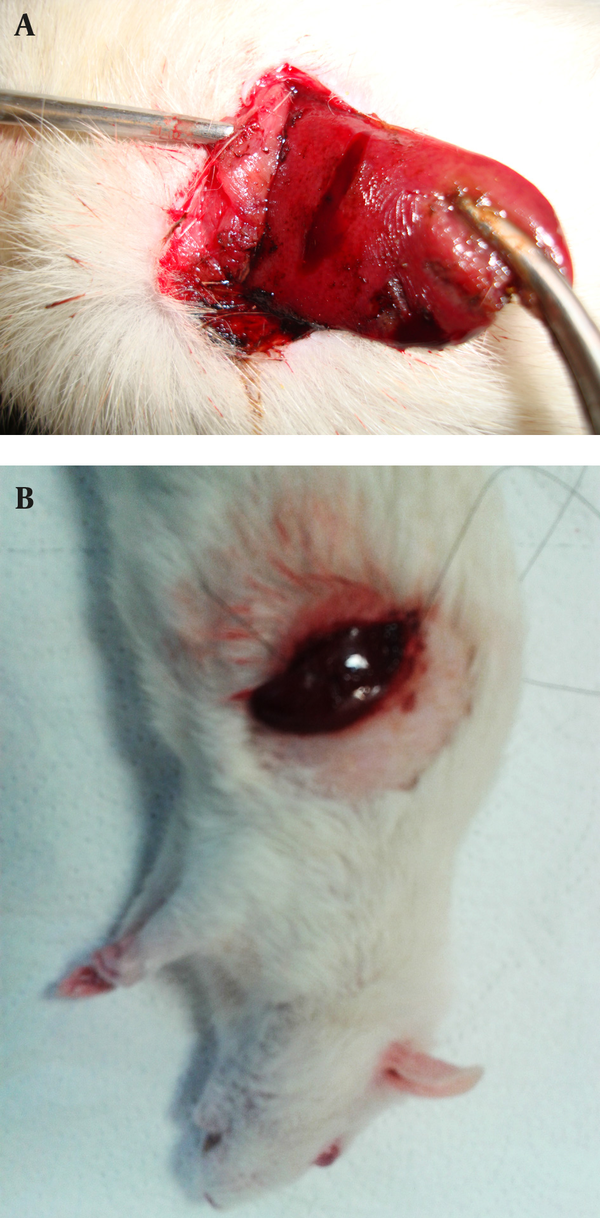
The alum was purchased from Merck (Darmstadt, Germany). Aqueous solution of alum 50%, 25%, 15%, 10% and 5% (w/v) were prepared in distilled water. Half a milliliter volume of solutions were applied to the incision sites by an insulin syringe. In fact, each concentration of alum was used in one of the groups, and the times of hemostasis were measured by chronometer (Figure 2). In the current study, the haemostatic time was considered as the time required for stopping the bleeding with no blood discharge from the incision site. The mean of ten measured times was considered as the haemostatic time for each concentration. Suturing, the standard method, was used in the control group to be compared with the results of the alum concentrations. The time of liver hemostasis was measured using sutures and (all the stitches were made by the same surgeon) on the livers of the ten rats kept and fed similar to the other groups, and the mean of the ten obtained times was compared with the results of different concentrations of alum. After controlling liver hemorrhage, subcutaneous and skin were closed again, and to prevent infection, each rat received 50 mg of keflin through an intraperitoneal injection ((cefalotin 1 g/Vial), Aspen Pharmacare Australia Pty Ltd, Australia).
3.4. Pathological Study
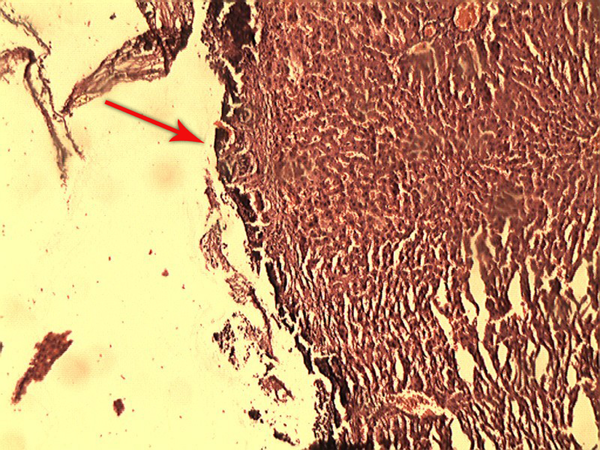
After one week, the rats were anesthetized by IP injection of a ketamine/xylazine mixture (ketamine 100 mg/kg and xylazine 10 mg/kg) and were placed in a supine position on the operating table. Then an incision was made on the previous site and the rats’ livers were resected, immediately placed and fixed in formalin, and sent to the laboratory for the pathological evaluations (Figure 3). Studying the pathological effect of alum on the liver tissue was performed through staining with haematoxylin and eosin (H and E) by light microscopy. Based on the defined pathological grading (13), the results were classified into six grades: zero = no change; one = minor inflammatory infiltration without edema; two = mild to moderate inflammatory infiltration with mild edema; three = mild to moderate inflammatory infiltration with moderate edema; four = moderate inflammation with neutrophils scattered and diffuse edema; five = severe inflammation of tissue and edematous changes, fibrosis, and hemorrhage.
3.5. Statistical Analysis
The SPSS software, version 16 was employed, and because of the abnormal distribution of variables in Kolmogorov-Smirnov test, the data was analyzed using Kruskal-Wallis test, Mann-Whitney, and also Wilcoxon Signed Ranks test.
Extraction of the Liver Lobe from the Abdominal Cavity
Control of the Liver Bleeding by (a) Alum 50% and (b) Suturing Technique
Pathological Effects of Alum on the Liver Tissue
4. Results
4.1. Haemostatic Results
The haemostatic time of the six groups are shown in Table 1. In all the groups complete hemostasis occurred, and there was a statistically significant difference between all groups (P < 0.001). The haemostatic times of different concentrations of alum were significantly less than that of the control group (P < 0.001).
4.2. Pathological Results
In 5%, 10% and 15% concentrations of the alum groups and the suture group, grade one was the most common pathological grade. In addition, in the 25% and 50% alum groups, pathological grade two was the most common grade. Grade zero, three, four, and five were not observed in any of the study groups (Table 2).
The Hemostasis Time Using Different Concentrations of Alum and Suturing Technique in Liver Parenchymaa
| Groups | Alum, 5% | Alum, 10% | Alum, 15% | Alum, 25% | Alum, 50% | Suture |
|---|---|---|---|---|---|---|
| Haemostatic times, Sec | 41.30 7.48 | 31.90 4.72 | 23.00 3.91 | 13.20 2.89 | 8.10 2.28 | 91.20 5.18 |
The Frequency of Liver Pathological Grade After Exposure to Different Concentrations of Alum and Suturing Techniquea
| Pathological grade | Groups | |||||
|---|---|---|---|---|---|---|
| Alum, 5% | Alum, 10% | Alum, 15% | Alum, 25% | Alum, 50% | Suture | |
| Grade 1 | 10 (100) | 10 (100) | 10 (100) | 6 (60) | 4 (40) | 10 (100) |
| Grade 2 | 0 | 0 | 0 | 4 (40) | 6 (60) | 0 |
5. Discussion
Results of the current study showed that, compared with the standard method used in the control group (with a deep suture of the liver parenchyma) alum needs significantly less time to exert its haemostatic effect. Currently, the choice techniques used to minimize bleeding during liver surgery are based on personal preference, physicians’ experience and facilities available in the treatment centers. The standard method is used to control bleeding of the liver lacerations clamps in the vascular area by deep stitches or the pack method (14).
It should be considered that liver bleeding control with sutures could cause more injuries, both parenchymal and ischemic, in the normal liver tissues. On the other hand, the liver parenchymal tissue is not suitable for stitching, and with a low-experienced surgeon, the sutures will exacerbate the rupture of the liver parenchyma. Pack method also has the risk of re-bleeding and abdominal compartment syndrome, which imposes additional surgery to the patient. Intermittent clamping of the portal triad is also associated with more bleeding than continuous clamping (5).
Local agents, such as fibrin sealants, provide a matrix for endogenous coagulation, and stimulate haemostasis on the cut surface of the liver parenchyma. In fact, to exert their functions they require normal homeostatic systems, which is a big disadvantage of this drug class, because many of the issues such as cirrhosis that require surgery of the liver due to liver dysfunction impair the hemostatic function of the body (15). In a large randomized controlled trial in 300 patients undergoing partial liver resection, Figueras et al. found no difference in the total blood loss, transfusion requirements, or postoperative morbidity between patients treated with fibrin sealants (n = 150) and a control group without fibrin sealants (n = 150) (16). Aprotinin and tranexamic acid caused a significant reduction in the blood loss and transfusion requirements around 30% to 40%. Due to the recent safety concerns, especially a high risk of renal failure and perioperative death in patients given aprotinin during cardiac surgery, marketing of aprotinin has been recently suspended (17).
Few studies have been conducted on local haemostatic agents to indicate the efficiency of these materials in reducing haemostatic time, the patients’ need for blood and blood products transfusion, and leading to improved prognosis of patients after a liver surgery (18, 19). Alum, unlike the well-known haemostatic agents, exerts its haemostatic effect through a chemical reaction with blood, and this property makes alum a very efficient haemostatic agent to exert its effect without the need to the normal body haemostatic agents (10-12). Besides, the acidic property of alum should be considered too; this chemical agent, reacting with blood proteins, can create a barrier by the coagulated proteins to prevent the outflow of blood from vessels. On the other hand, it prevents the alum from entering the vessels, which will not allow potential systemic complications of alum such as alum-induced thrombosis in liver capillaries (10-12).
In the current study, after exposing the liver tissues to alum they were sent to the laboratory to evaluate the pathological effect of this haemostatic agent. In order to determine the inflammation of the liver, caused by exposure to the alum as a foreign body, a pathological grading was used. This grading was scored from zero to five, according to the severity of the inflammation. Pathological reports showed that the alum, even at very high concentration (50%) did not cause any inflammation greater than grade two, and the immune system reaction against this haemostatic agent did not differ much from the standard method (sutures) used to control superficial bleeding. In another study, Nouri et al. sought the haemostatic effect of ferric sulfate on external bleeding. They reported that ferric sulfate was an effective haemostatic agent, and noted that skin tissue of Wistar rats had a slight inflammatory reaction to alum as a foreign body (20).
Kim and Rethnam stated that a good haemostatic material is the one that stops bleeding in the shortest possible time, one that is easily portable and compatible with life, imposes minimum complications to the patient, does not interfere with tissue healing and has a reasonable price (13). Considering the definition of a haemostatic agent provided by these researchers, the unique mentioned features of alum, such as not requiring normal haemostatic system for function, unlike other haemostatic agents, make this chemical substance an extremely effective topical haemostatic agent to control liver parenchymal tissue bleeding, compared to the other methods.
Acknowledgements
References
-
1.
Holt DR, Thiel DV, Edelstein S, Brems JJ. Hepatic resections. Arch Surg. 2000;135(11):1353-8. [PubMed ID: 11074895].
-
2.
Jarnagin WR, Gonen M, Fong Y, DeMatteo RP, Ben-Porat L, Little S, et al. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg. 2002;236(4):397-406. discussion 406-7. [PubMed ID: 12368667]. https://doi.org/10.1097/01.SLA.0000029003.66466.B3.
-
3.
Makuuchi M, Sano K. The surgical approach to HCC: our progress and results in Japan. Liver Transpl. 2004;10(2 Suppl 1):S46-52. [PubMed ID: 14762839]. https://doi.org/10.1002/lt.20044.
-
4.
Clark WR, Jr, Leather RP. Hemostasis during liver resections. Surgery. 1970;67(3):556-7. [PubMed ID: 5413451].
-
5.
Helewski K, Kowalczyk-Ziomek G, Czecior E, Wyrobiec G, Harabin-Slowinska M, Juszko-Piekut M, et al. Protective effect of intermittent clamping of the portal triad in the rat liver on liver ischemia-reperfusion injury. Hepat Mon. 2011;11(6):445-51. [PubMed ID: 22087176].
-
6.
Bechstein WO, Neuhaus P. [Bleeding problems in liver surgery and liver transplantation]. Chirurg. 2000;71(4):363-8. [PubMed ID: 10840602].
-
7.
Cogbill TH, Moore EE, Jurkovich GJ, Feliciano DV, Morris JA, Mucha P. Severe hepatic trauma: a multi-center experience with 1,335 liver injuries. J Trauma. 1988;28(10):1433-8. [PubMed ID: 3172301].
-
8.
Beal SL. Fatal hepatic hemorrhage: an unresolved problem in the management of complex liver injuries. J Trauma. 1990;30(2):163-9. [PubMed ID: 2304109].
-
9.
David Richardson J, Franklin GA, Lukan JK, Carrillo EH, Spain DA, Miller FB, et al. Evolution in the management of hepatic trauma: a 25-year perspective. Ann Surg. 2000;232(3):324-30. [PubMed ID: 10973382].
-
10.
Nouri S, Sharif MR. Efficacy and safety of ferric chloride in controlling hepatic bleeding; an animal model study. Hepat Mon. 2014;14(6). [PubMed ID: 24976842]. https://doi.org/10.5812/hepatmon.18652.
-
11.
Smith NL, Seale NS, Nunn ME. Ferric sulfate pulpotomy in primary molars: a retrospective study. Pediatr Dent. 2000;22(3):192-9. [PubMed ID: 10846729].
-
12.
Park JH, Kim MR, Kim DY. A philological study on poisoning of Alumen. J East-West Med. 2008;33:13-23.
-
13.
Kim S, Rethnam S. Hemostasis in endodontic microsurgery. Dent Clin North Am. 1997;41(3):499-511. [PubMed ID: 9248687].
-
14.
Carrillo EH, Richardson JD. The current management of hepatic trauma. Adv Surg. 2001;35:39-59. [PubMed ID: 11579817].
-
15.
Boonstra EA, Molenaar IQ, Porte RJ, de Boer MT. Topical haemostatic agents in liver surgery: do we need them? HPB (Oxford). 2009;11(4):306-10. [PubMed ID: 19718357]. https://doi.org/10.1111/j.1477-2574.2009.00065.x.
-
16.
Figueras J, Llado L, Miro M, Ramos E, Torras J, Fabregat J, et al. Application of fibrin glue sealant after hepatectomy does not seem justified: results of a randomized study in 300 patients. Ann Surg. 2007;245(4):536-42. [PubMed ID: 17414601]. https://doi.org/10.1097/01.sla.0000245846.37046.57.
-
17.
Berrevoet F, de Hemptinne B. Use of topical hemostatic agents during liver resection. Dig Surg. 2007;24(4):288-93. [PubMed ID: 17657154]. https://doi.org/10.1159/000103660.
-
18.
Heaton N. Advances and methods in liver surgery: haemostasis. Eur J Gastroenterol Hepatol. 2005;17 Suppl 1:S3-12. [PubMed ID: 15894872].
-
19.
Chapman WC, Clavien PA, Fung J, Khanna A, Bonham A. Effective control of hepatic bleeding with a novel collagen-based composite combined with autologous plasma: results of a randomized controlled trial. Arch Surg. 2000;135(10):1200-4. discussion 1205. [PubMed ID: 11030881].
-
20.
Nouri S, Amirbeigy M, Hosseinpour M, Abdorrahim K, Sharif MR, Amirbeigy MK. Evaluation of the Hemostatic Effect of Alum in Controlling External Bleeding in Mouse at Kashan University of Medical Sciences, 2012. Iran J Surg. 2013;21:21-9.