1. Background
Serous Otitis Media (SOM) is one of the most common childhood illnesses. It refers to persistent fluid accumulation in the middle ear without any pain, fever, and redness of the ear canal (1-4). In the United States, SOM is developed in about 90% of children before school age, mainly from four months to six years of age, but its prevalence decreases after the age of six years (5). Moreover, SOM is the most common cause of referral for pediatric surgery in England (1-4).
The pathogenesis of SOM is unclear. It is a multifactorial disease in which anatomical, immunological, genetic, and environmental factors are involved. It is generally accepted that eustachian tube dysfunction plays a key role in the development of SOM at all ages. Adenoid hypertrophy is a common finding in patients with SOM (6). The risk of SOM is associated with the inhalation of cigarette smoke, bottle feeding, low socioeconomic status, male gender, low age, seasonal conditions, staying in daycare, and exposure to a large number of other children (1, 3, 7). The prevalence of SOM is considerably higher (about 60% - 85%) in patients with Down syndrome or cleft palate (3). Children with SOM usually have hearing impairments and speech problems. Conductive hearing loss is usually between 15 and 45 dB and is often detected when parents are concerned with the hearing-related behavior, school performance, or speech development of the child (1). Serous otitis media have severe negative impacts on children’s development, such as hearing loss, which is accompanied by long-term effects on speech and language, poor school performance, loss of social skills, reduced quality of life, and balance disorders (8). Although half of the children with SOM normally recover over three months and 95% of them over a year, some children do not recover and have persistent incurable or repeated problems, eventually requiring surgery. Tympanic perforation, tympanosclerosis, otorrhea, and cholesteatoma often occur in children with SOM (1). Every year, about 2.2 new cases are detected in the United States, with a cost of over 4 billion USD. However, indirect costs of SOM are higher because the disease is often asymptomatic and therefore, is not detected (3).
Several studies have reported a relationship between vitamin D deficiency and respiratory infections in children (9-17). This can be due to the presence of vitamin D receptors in immune cells, especially antigen producing cells, including active T and B lymphocytes, active macrophages, and dendritic cells. The chemotactic and phagocytic properties of macrophages and monocytes may increase in the presence of vitamin D, thus promoting their microbicidal properties. These effects have attracted attention to the role of vitamin D in the regulation of the immune system (18-21). Several studies have highlighted a relationship between low blood levels of vitamin D and diseases of ear, nose, and throat, such as otitis media (22-28). In Iran, a cross-sectional study and a case-control study examined the level of vitamin D in patients with SOM. The first study found no relationship between vitamin D deficiency and SOM in children of 2 - 7 years when seasonality was considered a confounding factor. In the second study, the difference in vitamin D levels was not statistically significant between the two groups of children aged 3 - 10 years (27, 29).
2. Objectives
This study aimed to examine the relationship between SOM and vitamin D in patients with adenoid hypertrophy based on age category.
3. Methods
The present cross-sectional study included 92 patients who attended the ENT Clinic of Ali Asghar Hospital in Tehran from November 2017 to September 2018. The subjects were selected through a non-probability convenience sampling method. After collecting the data, three patients were excluded due to incomplete information, and the study was performed on the remaining 89 patients.
The total sample size was calculated as 86, including 43 patients in each group, based on the formula for comparing two means considering the alpha error of 0.05, the beta error of 0.1, and d of 3.8. The sample size was also determined according to the study by Asghari et al. (27), who suggested standard deviations of 4.7 and 6.3 for the SOM and control groups.
The inclusion criteria were the age range of 1 - 15 years, the history and symptoms of upper airway obstruction (open mouth, snoring, restlessness during sleep, nasal speech, frequent colds, sleep apnea, enuresis, and adenoid face), and adenoid hypertrophy grades 3 and 4 in the adenoid view. Moreover, the exclusion criteria were asthma, congenital and skeletal malformations such as nasal septum deviation, and underlying diseases such as cystic fibrosis and diabetes.
The demographic data and the results of history, examination, radiography, tympanometry, and serum vitamin D levels were recorded in researcher-made checklists. To this end, all subjects meeting the inclusion criteria underwent physical examinations. Moreover, tympanometry (gold standard) and the serum levels of 25 (OH) vitamin D were measured in 5 mL of venous blood through ELISA by spectrophotometry and recorded in the checklists. Vitamin D levels of < 20 ng/mL, 20 - 32 ng/mL, and 32 - 100 ng/mL were considered as deficient, insufficient, and adequate, respectively (30). Type A tympanogram was accepted as normal, and types B and C as SOM (3, 31). The checklist data were entered into SPSS V. 22, and the relationship between SOM and vitamin D deficiency was studied. Descriptive analysis was performed using the frequency, mean, and standard deviation. The mean serum levels of vitamin D were compared between the groups through the Independent-sample t-test, one-way ANOVA, and chi-square test. A P value of < 0.05 was considered statistically significant. The study followed the basic principles of the Helsinki Declaration (the ethics of medical research on humans) and the Ethics Committee of Iran University of Medical Sciences (IR.IUMS.FMD.REC1396.9421710007). Informed consent was obtained from the parents of the children.
4. Results
This study was performed on 89 children of 1 - 15 years with adenoid hypertrophy who met the inclusion criteria. The mean age of the patients was 69.9 ± 25.6 months, and there were 47 (52.8%) boys and 42 (47.2%) girls. According to tympanometry, 25 boys and 21 girls had SOM.
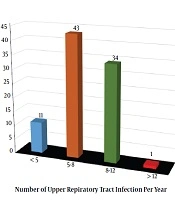
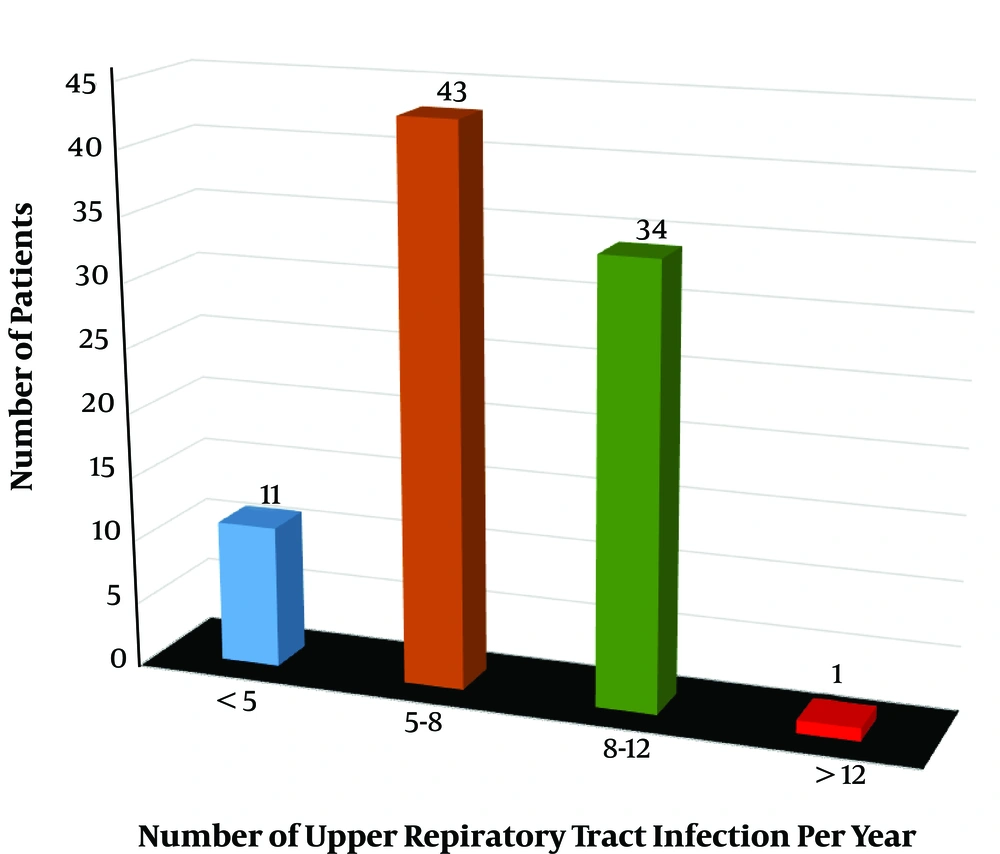
The complaints and symptoms at admission in order of frequency included open mouth breathing, snoring, restlessness during sleep, nasal speech, frequent colds, sleep apnea, enuresis, and adenoid face (98.9, 95.5, 94.4, 86.5, 68.5, 60.7, 12.4, and 7.9%, respectively). Besides, the number of patients based on the number of upper respiratory tract infections per year is illustrated in Figure 1.
The number of patients with and without SOM was 51 (57.3%) and 38 (42.7%) according to the initial physical examination and 46 (51.7%) and 43 (48.3%) according to tympanometry, respectively. There was a statistically significant relationship between the clinical examination and the tympanometry result (P < 0.001).
4.1. Comparison of Mean Serum Vitamin D Levels Based on Gender, Age, and SOM
The total mean serum vitamin D level was 23.86 ± 12.7 ng/mL (ranging from 8.1 ng/mL to 56.6 ng/mL). Moreover, the mean serum vitamin D level was 24.7 ± 13.1 ng/mL in children of 1 - 15 years with adenoid hypertrophy who had SOM based on tympanometry and 22.9 ± 12.2 ng/mL in patients without SOM although the difference was not statistically significant given the P value of 0.496 (Table 1). Besides, the mean serum vitamin D level was 21.9 ± 11.5 ng/mL in boys and 26 ± 13.7 ng/mL in girls, with no statistically significant difference (P = 0.13) (Table 1). Finally, the mean serum vitamin D level in all cases and controls was 24.1 ± 12.88 ng/mL in the age group of < 84 months and 23.3 ± 12.4 ng/mL in the age group of > 84 months, but the difference was not statistically significant (P = 0.79) (Table 1).
| Vit D Level (ng/mL)a | P Value | |
|---|---|---|
| Gender | 0.13 | |
| Male | 21.9 ± 11.5 | |
| Female | 26 ± 13.7 | |
| Age (months) | 0.79 | |
| < 84 | 24.1 ± 12.88 | |
| > 84 | 23.3 ± 12.4 | |
| SOM (based on tympanogram) | 0.496 | |
| Yes | 24.7 ± 13.1 | |
| No | 22.9 ± 12.2 |
aValues are expressed as mean ± SD.
4.2. Comparison of Serum Vitamin D Levels and Tympanometry Results According to Age and Gender
According to tympanometry, 36 (60%) out of 60 patients suffered from SOM in the age group of < 84 months while only 10 (35%) out of 29 patients had SOM in the age group of > 84 months, indicating a statistically significant difference between these values (P = 0.024). The mean serum vitamin D level was 24 ± 12.8 ng/mL in male patients with adenoid hypertrophy who had SOM based on tympanometry and 19.5 ± 9.5 ng/mL in patients without SOM although the difference was not significant given the p-value of 0.18 (Table 2). Further, the mean serum vitamin D level was 25.6 ± 13.75 ng/mL in female patients with adenoid hypertrophy who had SOM based on tympanometry and 26.5 ± 13.88 ng/mL in patients without SOM, but the difference was not meaningful given the p-value of 0.84 (Table 2).
| Vit D Level (ng/mL) | SOM (Based on Tympanogram)a | P Value | |
|---|---|---|---|
| Yes | No | ||
| Gender | |||
| Male | 24.1 ± 12.8 | 19.5 ± 9.5 | 0.18 |
| Female | 25.6 ± 13.8 | 26.5 ± 13.9 | 0.84 |
| Age (months) | |||
| < 84 | 26.85 ± 13.5 | 19.99 ± 10.87 | 0.04 |
| > 84 | 17.2 ± 8.4 | 26.58 ± 13.1 | 0.05 |
aValues are expressed as mean ± SD.
Given that eustachian tubes evolve at the age of seven, the study population was divided into two groups of 60 patients with the age of < 7 years (< 84 months) and 29 patients with the age of > 7 years (> 84 months). In the age group of < 84 months, 24 patients with adenoid hypertrophy without SOM had the mean serum vitamin D level of 19.99 ± 10.87 ng/mL while 36 patients with adenoid hypertrophy and SOM had the mean serum vitamin D level of 26.85 ± 13.5 ng/mL; the difference was observed to be statistically significant (P = 0.04) (Table 2).
Moreover, in the age group of > 84 months, the mean serum vitamin D levels were 26.58 ± 13.1 ng/mL in 19 patients with adenoid hypertrophy without SOM and 17.2 ± 8.4 ng/mL in 10 patients with adenoid hypertrophy and SOM, which were almost significantly different (P = 0.05). However, this difference was opposite to that in children in the age group of < 84 months with adenoid hypertrophy without SOM who had a lower mean serum vitamin D level (Table 2).
5. Discussion
The auditory system plays a crucial role in balance, path-finding, learning, and briefly, in human development. Therefore, any factor that adversely affects the functioning of this system can irreparably damage human development. Serous otitis media, due to its high prevalence at an early age, which is a period for learning and development, can quietly and insidiously affect the hearing system, resulting in irreparable consequences or surgical interventions.
The present study was designed to investigate the role of vitamin D deficiency in the incidence of SOM among children aged 1 - 15 years in total and based on age, as eustachian tubes evolve at the age of seven (84 months). The study was performed among children with adenoid hypertrophy, attempting to evaluate the possible role of vitamin D deficiency in the incidence of SOM through measuring and comparing vitamin D levels in children with and without SOM in two age groups. Several studies have investigated the relationship of low levels of vitamin D with acute otitis media and SOM (27, 29-32). Some studies have shown similar results in the relationship between SOM and vitamin D without considering age category. For instance, Asghari et al. conducted a cross-sectional study on 74 children of 2 - 7 years. They observed that of the population, 32 were diagnosed with SOM and considered as candidates for myringotomy or VT insertion, whereas the other 42 were regarded as candidates for tonsillectomy as the control group. There was a statistically significant difference in the 25-OH vitamin D level between patients admitted in winter (9.04 ± 2.94 ng/mL) and those admitted in summer (19.85 ± 4.21) (P = 0.001). However, the role of vitamin D was not significant in the development of OME in patients with adenotonsillar hypertrophy when seasonality was considered a confounding factor (27). In addition, in a case-control study, Hosseini et al. compared serum vitamin D levels in 120 children aged 3 - 10 years with and without SOM (control group), who were candidates for tonsillectomy. They reported the mean serum vitamin D levels of 26.1 ± 14.6 ng/mL in the SOM group and 29.5 ± 17.9 ng/mL in the control group. Although the mean serum vitamin D level was lower in the SOM group, the difference between the two groups was not statistically significant (P = 0.27) (29). Furthermore, a case-control study was carried out in New Zealand in 2013 on 3 - 4-year-old children, including 178 children with chronic SOM, who were candidates for tympanostomy, and 179 healthy children. The results showed that the mean serum vitamin D levels were 28.67 ± 8.9 ng/mL in children with SOM and 29.67 ± 10.6 ng/mL in healthy children, although there was no statistically significant difference between the groups (P = 0.34) (32). Yet, another study by Marchisio et al. on 116 Italian children under five years with Recurrent Acute Otitis Media (RAOM) demonstrated that vitamin D supplementation reduced the risk of RAOM (P = 0.03) (22).
As opposed to the above results, Akcan et al. conducted a prospective, controlled study in Turkey on children of 1 - 13 years, including 174 patients with SOM and 80 patients without SOM. After three months of follow-up, the level of vitamin D was measured as 18.98 ± 10.60 ng/mL in the SOM group and 28.07 ± 14.10 ng/mL in the control group without SOM, which showed a significant difference (P < 0.001) (31). Moreover, in a study by Cayir et al. in Turkey on children aged 1 - 5 years, including 84 children with recurrent Acute Otitis Media (AOM) and 108 healthy children, the mean serum vitamin D levels were 11.4 ± 9.8 ng/mL in the case group and 29.4 ± 13.9 ng/mL in the control group, and the difference between the groups was statistically significant (P < 0.05) (30).
In the present study, based on tympanometry, the mean serum vitamin D levels were 24.7 ± 3.1 ng/mL in children with SOM and 22.9 ± 12.2 ng/mL in children without SOM although the difference was not statistically significant regardless of their age (P = 0.496). Limiting the inclusion criteria to patients with adenoid hypertrophy was the main difference between the current research and other studies. This study suggests that adenoid hypertrophy is a more important factor than the vitamin D level in the development of SOM. As a remarkable point, children were divided into two age groups of < 7 years (< 84 months) and > 7 years according to the age at which eustachian tubes were developed. As observed, the mean serum vitamin D level was low in children without SOM in the age group of < 84 months based on tympanometry (19.99 ± 10.87 ng/mL) but high in children without SOM (26.85 ± 13.5 ng/mL), with a statistically significant difference (P = 0.04). This difference cannot be scientifically justified and may arise from high attention paid to children with SOM and the higher number of visits these children received from physicians, resulting in the earlier treatment of vitamin deficiency. However, in the age group of > 84 months, the mean serum vitamin D level was lower in children with SOM based on tympanometry than in those without SOM (17.2 ± 8.4 ng/mL versus 26.58 ± 13.1 ng/mL), and there was a significant difference between these values (P = 0.05). This significant difference indicates factors other than adenoid hypertrophy to play a role in older patients when eustachian tubes are developed completely.
Therefore, the age should be considered a determining factor in the development of SOM. Indeed, risk factors for serious otitis change by increasing age. One of the strengths of this study is the classification of patients based on age. It distinguishes our study from previous studies. However, we faced some limitations. First, the cross-sectional design of the study distorted the establishment of a causal relationship. Second, we attempted to consider some potential confounders as the exclusion criteria, but it is proposed for future studies to consider seasonality as a confounding variable due to its impact on the vitamin D level.
Given the prevalence of abnormal vitamin D level among Iranian children and its role in the incidence of middle ear diseases and upper respiratory tract infections, it is proposed to conduct a larger research project with higher sample size considering the age category, based on the age of eustachian tube development, to determine the status of serum vitamin D level and related risk factors in Iran.
5.1. Conclusions
Vitamin D has an extremely subtle role in increasing the incidence of SOM in patients with adenoid hypertrophy, especially in children younger than seven years of age. Moreover, adenoid hypertrophy plays a major role in the onset of serous otitis. However, in older children, other factors, such as vitamin D deficiency, can be effective in the development of SOM. Therefore, timely diagnosis and treatment of vitamin D deficiency in these children can prevent the onset of SOM and reduce family and health system costs.