Abstract
Background:
Since the incidence of food adulteration is rising, finding a rapid, accurate, precise, low-cost, user-friendly, high-throughput, ruggedized, and ideally portable method is valuable to combat food fraud. Near-infrared spectroscopy (NIRS), in combination with a chemometrics-based approach, allows potentially rapid, frequent, and in situ measurements in supply chains.Methods:
This study focused on the feasibility of a benchtop Fourier-transformation-NIRS apparatus (FT-NIRS, 1000 - 2500 nm) and a portable short wave NIRS device (SW-NIRS, 740 - 1070 nm) for the discrimination of genuine and citric acid-adulterated lime juice samples in a cost-effective manner following chemometrics study.Results:
Principal component analysis (PCA) of the spectral data resulted in a noticeable distinction between genuine and adulterated samples. Wavelengths between 1100 - 1400 nm and 1550 - 1900 nm were found to be more important for the discrimination of samples for the benchtop FT-NIRS data, while variables between 950 - 1050 nm contributed significantly to the discrimination of samples based on the portable SW-NIRS data. Following partial least squares discriminant analysis (PLS-DA) as a discriminant model, standard normal variate (SNV) or multiplicative scatter correction (MSC) transformation of benchtop FT-NIRS data and SNV in combination with the second derivative transformation of portable SW-NIRS data on the training set delivered equal accuracy (94%) in the prediction of the test set. In the soft independent modeling of class analogy (SIMCA) as a class-modeling approach, the overall performances of generated models on the auto-scaled data were 98% and 94.5% for benchtop FT-NIRS and portable SW-NIRS, respectively.Conclusions:
As a proof of concept, NIRS technology coupled with appropriate multivariate classification models enables fast detection of citric acid-adulterated lime juices. In addition, the promising results of portable SW-NIRS combined with SIMCA indicated its use as a screening tool for on-site analysis of lime juices at various stages of the food supply chain.Keywords
Adulteration Chemometrics Citric Acid Benchtop FT-NIR, Lime Juice Portable SW-NIR
1. Background
Food adulteration or “food fraud” is defined as the intentional substitution of food with inferior substances, removal of some valuable compounds, and misrepresentation of food ingredients for financial advantages and economic motivation (1). Food fraud lowers food quality, has a significant economic impact, and carries incidentally public health threats (2, 3). Over the past decades, several major adulterated cases in agro-food products have been discovered. For instance: The Chinese milk scandal where the milk products and infant formula were adulterated with melamine (4, 5), the contamination of chili powder with dye (6), several cases of the adulteration of spices with ground materials (7), the Irish pork crisis (8), the horsemeat scandal (9), adulteration of olive oil with hazelnut oil (10) and honey made from an artificial sweetener (11) are just some examples. Besides, several cases of adulteration in fruit juices have been detected in recent years (12, 13).
Lime and lemon, two main citrus family members, are commercialized as fresh fruits and juices (14). Lime juice is highly prone to adulteration by unscrupulous producers due to growing consumers demand all over the world. Most often, adulteration in lime juice happens by water dilution and subsequent addition of citric acid, sugars, pulp wash, cheaper ingredients, and non-recommended minor compounds to compensate for flavor and odor loss and even sometimes to prepare completely synthetic products (15). Citric acid concentration is the main factor affecting the price of lime juice. Thus, adding exogenous citric acid could be considered one of the most likely types of adulterations in lime juice.
Detection of adulterated raw materials or finished products is an important issue for official bodies in charge of labeling and governmental organizations where imported batches from abroad must be tested for compliance with specifications (16). It is also pivotal for control by businesses in the supply chain. Several methods and techniques such as chromatographic analysis, mass spectrometry (MS)-based methods, electrophoretic methods, spectroscopic methods, and immunoassays have been utilized to detect adulteration and fraud in food products (17). Previously, high-performance liquid chromatography (HPLC) and isotope ratio mass spectroscopy (IRMS) techniques were used to detect adulterated lime and lemon juice samples. Although these techniques have high resolution, high sensitivity, and specificity, they are often technically challenging, expensive, labor and resource-intensive, and need large consumable requirements (18, 19).
Following adulteration crises, producers, retailers, and food authorities developed a great demand for rapid, user-friendly, high-throughput, ruggedized, and ideally portable methods (20). Spectroscopic-based methods, including Fourier-transformation infrared spectroscopy (FT-IRS), near-infrared spectroscopy (NIRS), Fourier-transformation near-Infrared spectroscopy (FT-NIRS), and Raman spectroscopy have always been mentioned as nondestructive techniques that could be applied for rapid, on-line and continuous monitoring of the market without any or minimal sample preparation and solvent consumption (21-23). It is well-known that NIRS combined with chemometrics could be used to detect adulterants (24). Since a NIR spectrometer, like other fingerprinting techniques, produces several hundred to thousands of data points as a single measurement, data science approaches such as chemometrics are fundamental for interpreting the obtained data (25, 26). The knowledge of chemometrics is required to magnify the relevant information and lessen the undesirable information in the spectra without missing any important data (16, 27).
Although fruit juices are included in the top 10 food categories that are most at risk of food fraud (28), there are only a few studies based on the portable NIRS for the rapid detection of fruit juice adulteration. The ability of benchtop NIRS and chemometrics to detect synthetic lime juices was reported by Shafiee and Minaei (15). In our previous study, we revealed the capability of a portable NIRS (Tellspec®, 900 - 1700 nm) and chemometrics approach for the discrimination of genuine and citric-adulterated lime juices (29). However, despite the versatility of NIRS technology, there is no information on the performance of benchtop FT-NIRS (range 1000 - 2500 nm) and a portable short wave NIRS (SW-NIRS, range 740 - 1070 nm) technology in the detection of this type of adulteration. In addition, in most previous studies, discriminant analysis techniques were applied, while class modeling approaches seem to be more suitable in the case of food authenticity assessment.
2. Objectives
This study aimed to compare the performance between the benchtop FT-NIRS and portable SW-NIRS approaches in combination with discriminant analysis and class modeling techniques for the authenticity assessment of lime juice.
3. Methods
3.1. Reagents and Standards
Analytical grade citric acid, iso-citric acid, and citric acid-2,2,4,4-d4 were obtained from Sigma-Aldrich (St. Louis, MO, USA). HPLC grade methanol was purchased from Merck Co. (Darmstadt, Germany) with a purity of 99.8%. Purified water was prepared with a Milli-Q system (MA, USA).
3.2. Sample Collection and Preparation
A total of 16 authentic lime fruit samples (Citrus latifolia) originating from Jahrom (Fars Province, Iran) were directly acquired from the local market of Tehran, Iran. Samples were then authenticated by a botanist at the herbarium department of Shahid Beheshti University of Medical Sciences (Tehran, I.R. Iran). Juices (about 500 mL from 1 kg of fruit) were prepared using a cold press juicer machine (MCP 3500, Bosch, Germany). Since the NIR spectra are highly affected by the homogeneity of the sample (30), lime juices were carefully homogenized using an ultra-turrax homogenizer (T8; IKA, Staufen, Germany) and were stored in the freezer at -18°C until the analysis day. Furthermore, 28 lime juice samples were provided by the Iran Food and Drug Administration that were labeled as citric acid-adulterated samples. To verify the nature of the samples, the citric acid to iso-citric acid ratio was determined in triplicate in all samples using an LC-MS/MS method (31).
3.3. LC-MS/MS Measurement
The measurement of citric acid and iso-citric acid contents in lime juice samples was performed according to a validated method using an LC-MS/MS system (31). Briefly, 50 µL of each sample was mixed with 1400 µL of water and 50 µL of d4-citric acid as the internal standard. The prepared mixture was filtered through a 0.22 µm syringe filter before analysis. The measurement of organic acid contents was performed on a Waters Alliance 2695 HPLC system (Waters, Milford, MA, USA) coupled with electrospray ionization (ESI) triple quadrupole Quattro Ultima mass spectrometer (Waters-Micromass, Manchester, UK). An octadecyl-silica C18 column (250 mm × 4.6 mm × 5 µm, GL Science, Japan) with mobile phase methanol/formic acid 0.1% was used to separate organic acids. The injection volume, flow rate, column temperature, and run-time were adjusted at 15 µL, 0.4 mL min-1, 40°C, and 25 minutes, respectively. The MS/MS system was operated in the negative ionization mode. Capillary voltage, extractor, RF lens, source temperature, desolvation temperature, desolvation gas, and cone gas (nitrogen 99.99% purity) flow rates were 4.12 kV, 2 V, 0.1 V, 130°C, 350°C, 500 Lh-1, and 50 Lh-1, respectively (31).
3.4. Benchtop FT-NIRS Spectral Collection
An N-500 FT-NIR spectrometer (Buchi AG, Flawil, Switzerland) equipped with a tungsten halogen lamp, InGaAs detector, and six round glass cuvette holder (QX 2.0 mm, Hellma Analytics, Müllheim, Germany) with a path length of 2 mm was utilized for spectral acquisition. For each sample, triplicate diffuse reflectance spectra were recorded in the range of 4000 - 10.000 cm-1 (1000 - 2500 nm) on average at a 4-cm-1 sampling interval (1500 individual wavelengths) at room temperature. To calibrate the equipment, a built-in external reference (laboratory air) was measured before each series. Before multivariate analysis, the spectra (R) were converted to absorbance units using log (1/R) transformation, and the replicates of each sample were averaged.
3.5. Portable SW-NIRS Spectral Collection
For spectral analysis, a small and portable (just 35 g) short wave NIR spectrometer (SW-NIRS) SCiOTM version 1.1 (ConsumerPhysics Inc®) in the diffuse reflectance mode was used. The SW-NIR spectrometer was modified by applying a strip of yellow transparent tape (Kapton 5413-1/4” × 3 m, 3M Maplewood, MN, USA) on the device LED according to the manufacturer's instructions to prevent excess fluorescence with certain materials. The spectrometer contains a wide-band NIR illumination source with a corresponding detector (740 - 1070 nm) and was controlled by bluetooth using the smartphone app ‘SCiOLab’ (ConsumerPhysics, version 2.3.0 (iOS)). Calibration of the spectrometer was carried out before each series of analyses by applying the 99 % diffuse reflectance white reference built-in into the cover of the spectrometer. SW-NIR spectra of the samples were acquired by placing the bottom of a glass vial upright on the optical and illuminating part of the apparatus. The glass vials were shaken immediately before measurement to guarantee homogeneity. Three spectral replicates of each sample were taken in the spectral range of 740 - 1070 nm, and the average of acquired scans was used for further analysis.
3.6. Multivariate Data Analysis
Chemometrics and multivariate statistical analysis were performed to evaluate obtained NIR spectra using MATLAB software (The Mathworks, Natick, MA, USA). Because of the effect of many factors on spectroscopic data, such as light scattering, instrumental drift, and baseline shift caused by differences in particle size and physical properties of the samples, it is not possible to analyze raw data directly and without any pre-processing (16). Raw NIR data were subjected to several data pre-processing methods such as smoothing, auto-scaling, multiplicative scatter correction (MSC, mean), standard normal variate (SNV), first derivative, and second derivative (Savitzky-Golay, window: 15 pt) to correct the scattering and overlapping effects of the signal without affecting chemical information of the samples reflected in the NIR spectra. These pre-processing methods were chosen as they are the most commonly effective for removing NIR additive baseline effects and multiplicative scatter effects (32).
3.6.1. Principal Component Analysis
Principal component analysis (PCA) as an unsupervised pattern recognition method was performed to decompose large datasets into latent variables, extract any relevant and interpretable structure, and provide visual information regarding the distances or the similarities of the objects in a new space (33).
3.6.2. Partial Least Squares Discriminant Analysis
Partial least squares discriminant analysis (PLS-DA) as a discriminant model was built to classify genuine and adulterated samples (34). The model was validated by internal (leave-one-out cross-validation) and external validation by dividing the sample set into a training and a test set. For this purpose, the initial data set was divided into the training set (60% of samples; 9 genuine and 17 adulterated samples) and test set (40% of samples; 7 genuine and 11 adulterated samples) using the Kennard-stone maximal distance division method (35) following averaging of triplicate sample spectra. The training set was used for internal validation and optimization of the generated models by performing leave-one-out cross-validation. The cross-validation process consisted of excluding one sample in the data set, creating the model on the rest of the samples, and classifying the left-out sample by the model. This procedure was repeated until each sample was left out one at a time. The external validation was carried out on the test set. Sensitivity, specificity, accuracy, and precision were computed to evaluate the performance of the generated classification models. Moreover, the receiver operator characteristic (ROC) curves were plotted. In this study, sensitivity, specificity, and accuracy are defined as the percentage of correct predictions in adulterated samples, correct predictions in genuine samples, and the percentage of total correct predictions, respectively. The following equations (Equations 1-3) were used to calculate the performance of each parameter (36, 37):
In Equations 1-3: TP, true positive; TN, true negative; FP, false positive; FN, false negative.
3.6.3. Soft Independent Modeling of Class Analogy
Soft independent modeling of class analogy (SIMCA) as a class modeling technique based on PCA was applied to portable SW-NIR and benchtop FT-NIRS data. In this technique, genuine samples were modeled independently from adulterated samples based on boundaries built using Hotelling’s T2 and Q statistics. In the current study, we avoided dividing genuine and adulterated samples into training and test sets since there were a limited number of samples in the modeled group. In this case, all the genuine samples were used as the training set for the model development, and the leave-one-out cross-validation method was employed during the model construction (38). All adulterated lime juice samples were used as the test set and were subjected to the developed model. To estimate the overall performance of the final model, the percentages of correctly assigned samples for the training set, cross-validation set, and adulterant test set were calculated (39).
4. Results and Discussion
4.1. Citric Acid to Iso-citric Acid Ratio
Following LC-MS/MS measurement of citric acid and iso-citric acid, samples were divided into two groups of genuine and adulterated based on the citric acid to iso-citric acid ratio. This ratio for each sample is presented in Table 1. According to the Association of the Industry of Juices and Nectars (AIJN) reference guideline for lime juice, samples with citric acid to iso-citric acid ratio over 300 are considered non-authentic (adulterated) samples (40).
Citric Acid to the Iso-citric Acid Ratio in the Genuine and Adulterated Samples a
| No. | Genuine Samples | Adulterated Samples |
|---|---|---|
| 1 | 135 ± 1 | > 1093 |
| 2 | 165 ± 3 | 432 ± 1 |
| 3 | 120 ± 2 | > 958 |
| 4 | 158 ± 1 | 439 ± 2 |
| 5 | 227 ± 2 | 935 ± 2 |
| 6 | 152 ± 3 | 433 ± 2 |
| 7 | 213 ± 1 | > 687 |
| 8 | 171 ± 2 | > 1405 |
| 9 | 166 ± 1 | > 1233 |
| 10 | 155 ± 4 | > 802 |
| 11 | 147 ± 2 | > 1412 |
| 12 | 109 ± 2 | > 1267 |
| 13 | 125 ± 3 | > 863 |
| 14 | 140 ± 2 | > 1460 |
| 15 | 151 ± 2 | > 1253 |
| 16 | 145 ± 2 | > 1258 |
| 17 | > 1407 | |
| 18 | 427 ± 3 | |
| 19 | 333 ± 1 | |
| 20 | 697 ± 2 | |
| 21 | 784 ± 2 | |
| 22 | 655 ± 2 | |
| 23 | > 1462 | |
| 24 | 749 ± 1 | |
| 25 | 445 ± 2 | |
| 26 | > 1472 | |
| 27 | > 1563 | |
| 28 | > 1605 |
4.2. Spectral Features
Each sample, either genuine or adulterated, was subjected to spectroscopic analysis by benchtop FT-NIRS and portable SW-NIRS in triplicate. The averaged spectrum were used for further data processing. Figure 1A presents the NIR spectra of homogenized lime juice samples acquired by the benchtop FT-NIRS in the range of 1000 - 2500 nm. Similarly, the NIR spectra of samples acquired by the portable SW-NIRS in the range of 740 - 1070 nm are shown in Figure 1B.
The median NIR spectra (solid lines) and the range between minimum and maximum intensity (shaded areas) obtained from lime juice samples in the benchtop FT-NIRS (boxed areas are excluded from further evaluation) (A); and portable SW-NIRS (B); SNV transformed spectra of the samples acquired in benchtop FT-NIRS (C); SNV in combination with second derivative transformed spectra of the samples acquired in portable SW-NIRS (D). FT-NIRS, Fourier-transformation near-infrared spectroscopy; SW-NIRS, short wave near-infrared spectroscopy; SNV, standard normal variate.
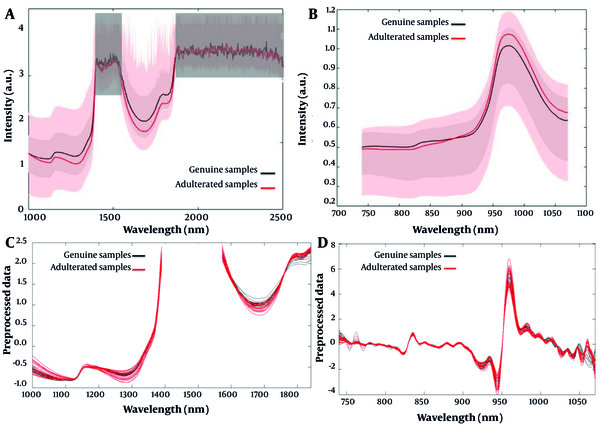
As shown in Figure 1A, in the benchtop FT-NIRS measurements, variables between 1100 - 1400 nm and 1550 - 1850 nm are more important for the discrimination of samples. The band from 1600 - 1800 nm could be related to the first stretching overtone of C-H and is likely associated with the organic acid composition of lime juice since organic acids have C-H bonds in their chemical structure. This finding agrees with the wavelength range used previously by Chen et al. for determining citric acid and malic acid content in Japanese apricot fruit juices (41). It was also reported by Bai et al. that in the wavelength region between 1400 - 2500 nm the absorptions owning to organic acids are sufficiently intense enough to be used for organic acids determination (42). Wavelengths around 1150 nm, 1380 nm, and 1550 nm were proposed by Xie et al. to have important roles in the calibration model of citric acid content (43). Furthermore, wavelengths around 1900 nm could be caused by the vibrational overtone of the C=O and C=OOH bands (44). Since the first stretching and the combination overtone of the O-H group are located in the wavelength region of 1400 - 1500 nm and 1950 - 2100 nm, respectively, the water content of fruit juices has strong absorption in these two wavelength ranges that contributes to the saturation of detector (45). Therefore, it is difficult to measure satisfactory spectra of organic acids in the mentioned regions (46). Moreover, low-energy light (longer wavelengths; spectra above 1900 nm) could not pass through the lime juice samples (15). Considering these issues, the mentioned regions were excluded from the benchtop FT-NIRS spectra for further data analysis since there was no reliable information (Figure 1A).
In Figure 1B, variables between 950-1050 nm significantly contribute to the discrimination of samples by portable SW-NIRS. As reported by Liu et al., the wavelength region between 950 - 1000 nm has a distinct effect on the determination of citric acid in the lemon vinegar samples (47). This region could be interpreted as being originated from the second overtone of the v(OH) stretching vibration (expected at 960 nm) (48). Moreover, the second and third overtones of O-H groups are present in the 700 - 800 and 900 - 1000 nm regions (45). Therefore, it could be concluded that the NIR absorbance spectrum in the defined range is affected by water content.
4.3. Principal Component Analysis
The averaged collected spectra of the samples were subjected to the PCA algorithm with different pre-processing techniques to check for possible cluster formations and outliers. SNV transforming of benchtop FT-NIRS data and SNV in combination with second derivative (Savitzky-Golay) for portable SW-NIR data resulted in the best separation of two groups in PCA. The pre-processed data are presented in Figure 1C and D. Since the lime juice samples are turbid, SNV correction was applied to cope with turbidity differences between the samples.
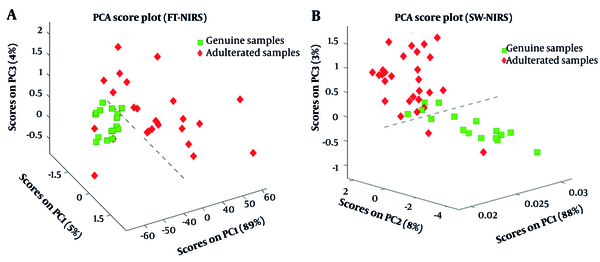
Figure 2A and B depict that the first three principal components (PCs) accounted for 98 % and 99% of data variance in benchtop FT-NIRS and portable SW-NIRS, respectively. PCA analysis of spectral data from benchtop FT-NIRS discriminates the genuine and adulterated lime juice samples according to their authenticity along with the first PC (Figure 2A), and the distinction between samples along the second PC is observed in the scores plot of portable SW-NIRS (Figure 2B). It can be concluded from unsupervised statistical analysis that the NIR spectra generated by both hardware variants contain enough chemical information to distinguish genuine from adulterated lime juices. Q residuals and Hotelling T2 distances were calculated per sensor and per type of sample to identify outliers (49). Three adulterated samples from FT-IR exceeded their respective 95% threshold and were considered outliers. In the SW-NIRS measurement, no outliers were identified.
Principal component analysis score plot of genuine and adulterated samples with PC1, PC2, and PC3 based on the data obtained from benchtop FT-NIRS (A); and portable SW-NIRS (B). Outliers were excluded from the plots. PC, principal component; FT-NIRS, Fourier-transformation near-Infrared spectroscopy; SW-NIRS, short wave near-infrared spectroscopy.
4.4. Partial Least Squares Discriminant Analysis
PLS-DA was used to discriminate samples into two classes using pre-processed spectral data. For the validation of data, internal validation (leave-one-out cross-validation) was applied. During internal validation, the optimal number of latent variables was selected to develop an efficient and robust model and to avoid over-fitting. Moreover, the model was externally validated by applying the generated model to the test dataset utterly independent of the training dataset. Quality metrics such as sensitivity (true positive rate), specificity (true negative rate), and accuracy were calculated following different pre-processing to evaluate the performance of the generated models on both benchtop FT-NIRS and portable SW-NIRS data. Furthermore, the area under the ROC curve (AUROC) was estimated to compare different models. Quality metrics in the training and validation sets of each model, as well as the number of LVs and the percent of explained variance, are presented in Table 2.
Quality Metrics for the Partial Least Squares Discriminant Analysis (PLS-DA) Models Generated Using the Training and Test Sets of Benchtop Fourier-Transformation Near-Infrared Spectroscopy (FT-NIRS) and Short Wave Near-Infrared Spectroscopy (SW-NIRS)
| Pre-processing (Number of LVs, Explained Variance) | Variables | Benchtop FT-NIRS (%) | Portable SW-NIRS (%) | ||
|---|---|---|---|---|---|
| Internal Validation (Training Set) | External Validation (Test Set) | Internal Validation (Training Set) | External Validation (Test Set) | ||
| Raw data | Sensitivity | 53 | 70 | 41 | 64 |
| FT-NIR (3, 100%) | Specificity | 56 | 83 | 67 | 57 |
| SW-NIR (2, 100%) | Accuracy | 54 | 75 | 50 | 61 |
| AUROC | 0.58 | 0.49 | |||
| Smooth | Sensitivity | 80 | 80 | 41 | 64 |
| FT-NIR (4, 100%) | Specificity | 67 | 86 | 67 | 57 |
| SW-NIR (2, 100%) | Accuracy | 75 | 82 | 50 | 61 |
| AUROC | 0.71 | 0.42 | |||
| 1st derivative | Sensitivity | 73 | 60 | 94 | 100 |
| FT-NIR (3, 99.40%) | Specificity | 67 | 100 | 67 | 100 |
| SW-NIR (4, 99.99%) | Accuracy | 71 | 76 | 85 | 100 |
| AUROC | 0.73 | 0.80 | |||
| 2nd derivative | Sensitivity | 67 | 80 | 81 | 100 |
| FT-NIR (3, 99.40%) | Specificity | 44 | 57 | 56 | 86 |
| SW-NIR (2, 99.89%) | Accuracy | 58 | 71 | 72 | 94 |
| AUROC | 0.59 | 0.82 | |||
| MSC | Sensitivity | 80 | 90 | 94 | 73 |
| FT-NIR (3, 100%) | Specificity | 100 | 100 | 67 | 86 |
| SW-NIR (3, 100%) | Accuracy | 88 | 94 | 85 | 78 |
| AUROC | 0.87 | 0.84 | |||
| SNV | Sensitivity | 80 | 90 | 94 | 73 |
| FT-NIR (3, 99.96%) | Specificity | 100 | 100 | 67 | 86 |
| SW-NIR (3, 99.99%) | Accuracy | 88 | 94 | 85 | 78 |
| AUROC | 0.87 | 0.84 | |||
| SNV + 2nd derivative | Sensitivity | 67 | 70 | 88 | 91 |
| FT-NIR (3, 95.26%) | Specificity | 56 | 57 | 100 | 100 |
| SW-NIR (3, 99.97%) | Accuracy | 63 | 65 | 92 | 94 |
| AUROC | 0.66 | 0.95 | |||
In the current study, the most accurate classification models were achieved by the SNV transformation of benchtop FT-NIRS data and SNV followed by the second derivative for portable SW-NIRS data, judged from the highest AUROC values in the internal validation set. However, the same accuracy was obtained following the MSC transformation of benchtop FT-NIRS data. MSC and SNV are two popular scatter correction techniques. Both are applied to eliminate all effects unrelated to the chemical nature of the sample, such as multiplicative effects related to the particle size of samples. Since the equations for applying the transformations have almost the same form, they often produce identical results (50).
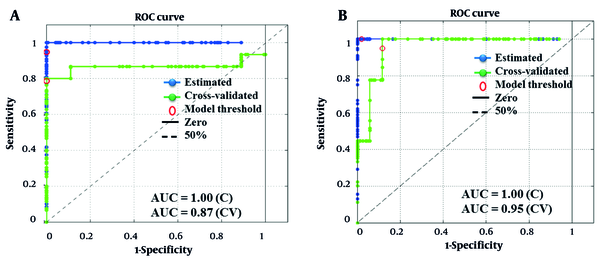
The PLS-DA model generated using the training set delivered an overall accuracy of 88% (benchtop FT-NIRS) and 92% (portable SW-NIRS) upon internal validation using leave-one-out cross-validation. The external validation set with unseen samples yielded comparable accuracies of 94% of both benchtop FT-NIRS and portable SW-NIRS data, confirming the capability of these models. Sensitivity depends on TP and FN, which are in the same column of the confusion matrix. Similarly, the specificity metric depends on TN and FP, which are in the same column; hence, both sensitivity and specificity can be used for the evaluation of classification performance with imbalanced data. Although accuracy is sensitive to imbalanced data, it considers all predictions and is a good identifier for the overall effectiveness of the classifier (37). As shown in Figure 3A and B, the ROC curve plots the TPR (sensitivity) of the model versus FPR (1-specificity). When the ROC curve reaches the top left corner, the sensitivity could be very good without losing specificity. AUROC-value of 0.5 represents a random decision method, whereas a good method represents an AUROC-value close to 1 (51). It can be observed from both curves that AUROC-value of 1 is achieved following estimation of samples, while upon cross-validation, the best models generated by benchtop FT-NIRS and portable SW-NIRS dataset produced the AUROC values of 0.87 and 0.95, respectively that indicate the adequate predictive capacity of generated models in the detection of adulterated lime juice samples.
The ROC plot of the generated models based on the benchtop FT-NIRS (A); and portable SW-NIRS (B) data. ROC, receiver operator characteristic; FT-NIRS, Fourier-transformation near-infrared spectroscopy; SW-NIRS, short wave near-infrared spectroscopy; C, calibration; CV, cross-validation.
From the results of internal and external validations on the limited sample sets, it can be concluded that both benchtop FT-NIRS data and portable SW-NIRS data in combination with the chemometrics approach have satisfactory performance in distinguishing citric acid-adulterated samples from genuine ones. This finding agrees with the results of our previous study on the efficacy of another portable NIRS device (Tellspec®, 900 - 1700 nm) for detecting citric acid-adulterated lime juice samples. The generated model on the Tellspec data was able to classify juices with an accuracy of 88% in the external validation set (29). Thus, it seems that NIRS could be considered a real alternative to the time and reagent-consuming methods for quality control of lime juice. Besides, portable NIRS has potential applicability across the fruit juice industry by conducting on-site sample analysis.
4.5. Soft Independent Modeling of Class Analogy
By using the SIMCA technique as a one-class classification model, we aimed to objectively classify samples into “genuine” and “non-genuine or adulterated” samples. Following the construction of each model, leave-one-out cross-validation was performed on the training set and the optimal number of latent variables selected. The percentage of correctly assigned samples for the training set, cross-validation set, adulterant test set, and overall performance of the constructed models following different pre-processing techniques are presented in Table 3. As illustrated in Table 3, auto-scaling of both benchtop FT-NIRS and portable SW-NIRS data resulted in the best performance of the models. In the mentioned models, 100% of genuine samples were correctly assigned in the cross-validation set of both benchtop FT-NIRS and portable SW-NIRS, while 96% and 89% of adulterated samples were correctly assigned in the test set of benchtop FT-NIRS and portable SW-NIRS, respectively.
The Results of Soft Independent Modeling of Class Analogy (SIMCA) Developed from Benchtop Fourier-transformation Near-Infrared Spectroscopy (FT-NIRS) and Portable Short Wave Near-Infrared Spectroscopy (SW-NIRS) Data
| Pre-processing (Number of LVs, Explained Variance) | Correctly Assigned Samples (%) | |||
|---|---|---|---|---|
| Training Set | Cross-Validation Set | Adulterated Test Set | Overall Performance | |
| Raw data | ||||
| FT-NIR (2, 100%) | 100 | 100 | 80 | 90 |
| SW-NIR (4, 100%) | 100 | 100 | 75 | 87.5 |
| Auto-scale | ||||
| FT-NIR (3, 100%) | 100 | 100 | 96 | 98 |
| SW-NIR (4, 100%) | 100 | 100 | 89 | 94.5 |
| Smooth | ||||
| FT-NIR (3, 100%) | 100 | 100 | 84 | 92 |
| SW-NIR (3, 100%) | 100 | 100 | 28 | 64 |
| 1st derivative | ||||
| FT-NIR (1, 99.50%) | 100 | 93 | 88 | 90.5 |
| SW-NIR (2, 99.99%) | 100 | 93 | 39 | 66 |
| 2nd derivative | ||||
| FT-NIR (1, 99.50%) | 100 | 93 | 88 | 90.5 |
| SW-NIR (1, 99.40%) | 100 | 93 | 39 | 66 |
| MSC | ||||
| FT-NIR (4, 100%) | 100 | 100 | 80 | 90 |
| SW-NIR (2, 100%) | 100 | 93 | 39 | 66 |
| SNV | ||||
| FT-NIR (4, 100%) | 100 | 100 | 80 | 90 |
| SW-NIR (4, 100%) | 100 | 93 | 89 | 91 |
| SNV + 2nd derivative | ||||
| FT-NIR (1, 98.56%) | 100 | 93 | 16 | 54.5 |
| SW-NIR (2, 99.50%) | 100 | 93 | 0 | 46.5 |
4.6. Comparison of Discriminant Analysis to the Class Modeling Approach
According to the accuracy obtained by the PLS-DA model and overall performance obtained by SIMCA, it seems that SIMCA (as a class modeling technique) could be more suitable in the authenticity assessment of lime juice samples compared to the PLS-DA (as a discriminant model). The class modeling approach should be adopted when the interest is focused on a single class, and the aim is to verify the compliance of other samples with the features of that target class, while discriminant analysis is the right choice when two classes are meaningfully defined (52). Although the ‘best’ classification method does not exist, PLS-DA is successfully used in ‘omics’ applications such as metabolomics and genomics, while SIMCA shows more reliable results in the authentication problems (53). The main drawback of discriminant methods is that they always assign a new sample to one of the predefined classes, even if that sample does not belong to any of those classes (54).
The results of our study also revealed that the one-class classification could be considered a suitable approach for authenticity assessment when there is a particularly small sample size. One of the most practical consequences of class modeling is that a model could be constructed with only data from the target class. This feature is not available in discriminant methods. When only a few genuine samples are available, dividing the whole data set (genuine samples and non-genuine samples) into training and test leads to training the model on an insufficient amount of data. In addition, the test set will not contain enough samples to sufficiently estimate the predictive capability of the generated models (55).
4.7. Comparison of the Performance of Benchtop FT-NIRS to Portable SW-NIRS
Although the effective wavelength ranges of benchtop FT-NIRS and portable SW-NIRS completely differ, both devices successfully classify lime juice samples. This is the first report comparing the performance of benchtop FT-NIRS and portable SW-NIRS in detecting lime juice adulteration. The outcome of this finding is that with a suitable classification model, good accuracies could be extracted from data, irrespective of the type of NIRS instrument used. Although the predictive performance of the portable SW-NIRS is lower relative to the benchtop FT-NIRS, the portable device presents adequate results for use as a screening technique. Considering the attributes of each instrument in terms of accuracy, cost, usability, and portability, both the benchtop FT-NIRS and portable SW-NIRS demonstrate an excellent analytical tool for on-site food integrity screening. Although the sample set employed in this study was relatively small, it fairly revealed the suitability of spectroscopic methods in the detection of this type of adulteration. However, more precise and robust models would need to be generated on a wider range of lime juice variability for a serviceable application.
Comparing the results of our study to others, Shafiee and Minaei demonstrated the potential of data mining combination with a VIS/NIR spectroradiometer (350 - 2500 nm) for evaluation of lime juice quality in terms of natural or synthetic nature. Based on the obtained results support vector machine (SVM) proved to be the most accurate classifier as it achieved the highest accuracy (97%) using the raw spectrum information (15). However, in our study, almost equal accuracy was obtained using a portable SW-NIRS. In our previous study, we revealed the capability of another portable NIRS (Tellspec®, 900 - 1700 nm) and chemometrics approach for the discrimination of genuine and citric-adulterated lime juices with the accuracy of 88% for each PLS-DA and k-NN models (29). In addition, the feasibility of FT-IR spectroscopy and chemometrics approach in the detection of adulterated lime juice (prepared by lime juice concentrates) was revealed in a study conducted by Mohammadian et al. The lime juice samples were correctly designated to their original groups using PLS-DA and counter propagation artificial neural networks (CPANN) maps with an overall accuracy of 87% in the validation procedure (12).
5. Conclusions
In this study, a novel NIRS method was developed for detecting citric acid-adulterated lime juices based on the NIR spectra of genuine and adulterated samples recorded by benchtop FT-NIRS and portable SW-NIRS. The results of the current study revealed that benchtop FT-NIRS and portable SW-NIRS with an appropriate multivariate classification model could be applied as quick, easy, and low-cost analytical approaches in the screening of lime juice samples. According to the accuracy obtained by the PLS-DA model and overall performance obtained by SIMCA, it seems that SIMCA (as a class modeling technique) is more appropriate in the authenticity assessment of lime juice samples compared to the PLS-DA (as a discriminant model). Since quality metrics for the benchtop FT-NIRS and the portable SW-NIRS were satisfactory, SW-NIRS as a portable device could be considered for future applications, moving out of the laboratory and on-site screening of a large number of samples by the food industries and regulators at various stages of the food supply chain. The suspected samples could be reconfirmed using confirmatory techniques. Although the sample set employed in this preliminary study was relatively small, the results proved that portable SW-NIRS is an appropriate method for the rapid and on-site detection of citric acid-adulterated lime juice samples. However, further experiments are required with considerably more samples to confirm the real potential of this technology and to develop more robust prediction models.
Acknowledgements
References
-
1.
Manning L, Soon JM. Food Safety, Food Fraud, and Food Defense: A Fast Evolving Literature. J Food Sci. 2016;81(4):R823-34. [PubMed ID: 26934423]. https://doi.org/10.1111/1750-3841.13256.
-
2.
Everstine K, Spink J, Kennedy S. Economically motivated adulteration (EMA) of food: common characteristics of EMA incidents. J Food Prot. 2013;76(4):723-35. [PubMed ID: 23575142]. https://doi.org/10.4315/0362-028X.JFP-12-399.
-
3.
Cavin C, Cottenet G, Blancpain C, Bessaire T, Frank N, Zbinden P. Food Adulteration: From Vulnerability Assessment to New Analytical Solutions. Chimia (Aarau). 2016;70(5):329-33. [PubMed ID: 27198809]. https://doi.org/10.2533/chimia.2016.329.
-
4.
Zhang M, Qiao H, Wang X, Pu MZ, Yu ZJ, Zheng FT. The third-party regulation on food safety in China: A review. J Integr Agric. 2015;14(11):2176-88. https://doi.org/10.1016/s2095-3119(15)61114-5.
-
5.
Yang Y, Huisman W, Hettinga KA, Zhang L, van Ruth SM. The Chinese milk supply chain: A fraud perspective. Food Control. 2020;113:107211. https://doi.org/10.1016/j.foodcont.2020.107211.
-
6.
Ou Y, Pei L, Lai K, Huang Y, Rasco BA, Wang X, et al. Rapid Analysis of Multiple Sudan Dyes in Chili Flakes Using Surface-Enhanced Raman Spectroscopy Coupled with Au–Ag Core-Shell Nanospheres. Food Anal Methods. 2017;10(3):565-74. https://doi.org/10.1007/s12161-016-0618-z.
-
7.
Silvis ICJ, van Ruth SM, van der Fels-Klerx HJ, Luning PA. Assessment of food fraud vulnerability in the spices chain: An explorative study. Food Control. 2017;81:80-7. https://doi.org/10.1016/j.foodcont.2017.05.019.
-
8.
Doherty E, Campbell D. Demand for safety and regional certification of food: Results from Great Britain and the Republic of Ireland. Br Food J. 2014;116(4):676-89. https://doi.org/10.1108/bfj-10-2011-0266.
-
9.
Agnoli L, Capitello R, De Salvo M, Longo A, Boeri M. Food fraud and consumers’ choices in the wake of the horsemeat scandal. Br Food J. 2016;118(8):1898-913. https://doi.org/10.1108/bfj-04-2016-0176.
-
10.
Georgouli K, Martinez Del Rincon J, Koidis A. Continuous statistical modelling for rapid detection of adulteration of extra virgin olive oil using mid infrared and Raman spectroscopic data. Food Chem. 2017;217:735-42. [PubMed ID: 27664692]. https://doi.org/10.1016/j.foodchem.2016.09.011.
-
11.
Wu L, Du B, Vander Heyden Y, Chen L, Zhao L, Wang M, et al. Recent advancements in detecting sugar-based adulterants in honey – A challenge. Trends Analyt Chem. 2017;86:25-38. https://doi.org/10.1016/j.trac.2016.10.013.
-
12.
Mohammadian A, Barzegar M, Mani-Varnosfaderani A. Detection of fraud in lime juice using pattern recognition techniques and FT-IR spectroscopy. Food Sci Nutr. 2021;9(6):3026-38. [PubMed ID: 34136168]. [PubMed Central ID: PMC8194754]. https://doi.org/10.1002/fsn3.2260.
-
13.
Xu L, Shi Q, Lu D, Wei L, Fu HY, She Y, et al. Simultaneous detection of multiple frauds in kiwifruit juice by fusion of traditional and double-quantum-dots enhanced fluorescent spectroscopic techniques and chemometrics. Microchem J. 2020;157:105105. https://doi.org/10.1016/j.microc.2020.105105.
-
14.
Khodadadi A, Nemati M, Tamizi E, Nazemiyeh H. Facile and Accelerated Method for Detection of Adulteration in Commercially Available Lime Juice Products in Iranian Marke. Pharm Sci. 2018;24(2):148-56. https://doi.org/10.15171/ps.2018.22.
-
15.
Shafiee S, Minaei S. Combined data mining/NIR spectroscopy for purity assessment of lime juice. Infrared Phys Technol. 2018;91:193-9. https://doi.org/10.1016/j.infrared.2018.04.012.
-
16.
Lohumi S, Lee S, Lee H, Cho BK. A review of vibrational spectroscopic techniques for the detection of food authenticity and adulteration. Trends Food Sci Technol. 2015;46(1):85-98. https://doi.org/10.1016/j.tifs.2015.08.003.
-
17.
Hong E, Lee SY, Jeong JY, Park JM, Kim BH, Kwon K, et al. Modern analytical methods for the detection of food fraud and adulteration by food category. J Sci Food Agric. 2017;97(12):3877-96. [PubMed ID: 28397254]. https://doi.org/10.1002/jsfa.8364.
-
18.
Bayati A, Nazari F, Hassanzadazar H, Hosseini MJ. Identification Parameters for Comparison of Naturally and Commercially Lime Juice. Carpathian J Food Sci Technol. 2020;12(1):110-9. https://doi.org/10.34302/crpjfst/2020.12.1.11.
-
19.
Mantha M, Kubachka KM, Urban JR, Dasenbrock CO, Chernyshev A, Mark WA, et al. Economically Motivated Adulteration of Lemon Juice: Cavity Ring Down Spectroscopy in Comparison with Isotope Ratio Mass Spectrometry: Round-Robin Study. J AOAC Int. 2019;102(5):1544-51. [PubMed ID: 30862326]. https://doi.org/10.5740/jaoacint.18-0401.
-
20.
Ellis DI, Muhamadali H, Haughey SA, Elliott CT, Goodacre R. Point-and-shoot: rapid quantitative detection methods for on-site food fraud analysis – moving out of the laboratory and into the food supply chain. Anal Methods. 2015;7(22):9401-14. https://doi.org/10.1039/c5ay02048d.
-
21.
Jamwal R, Kumari S, Sharma S, Kelly S, Cannavan A; Amit, et al. Recent trends in the use of FTIR spectroscopy integrated with chemometrics for the detection of edible oil adulteration. Vib Spectrosc. 2021;113:103222. https://doi.org/10.1016/j.vibspec.2021.103222.
-
22.
Qiu T, Yang Y, Sun H, Hu T, Wang X, Wang Y, et al. Rapid discrimination and quantification of kudzu root with its adulterant part using FT-NIR and a machine learning algorithm. Vib Spectrosc. 2021;116:103289. https://doi.org/10.1016/j.vibspec.2021.103289.
-
23.
Chen H, Tan C, Lin Z. The feasibility study of non-destructive detection of cashmere by near-infrared spectroscopy and data driven-based class-modeling. Vib Spectrosc. 2019;102:57-62. https://doi.org/10.1016/j.vibspec.2019.04.006.
-
24.
Chen H, Lin Z, Tan C. Fast quantitative detection of sesame oil adulteration by near-infrared spectroscopy and chemometric models. Vib Spectrosc. 2018;99:178-83. https://doi.org/10.1016/j.vibspec.2018.10.003.
-
25.
Pugner T, Knobbe J, Gruger H. Near-Infrared Grating Spectrometer for Mobile Phone Applications. Appl Spectrosc. 2016;70(5):734-45. [PubMed ID: 27170776]. [PubMed Central ID: PMC4871177]. https://doi.org/10.1177/0003702816638277.
-
26.
Mosaffaei Z, Jahani A, Chahouki MAZ, Goshtasb H, Etemad V, Saffariha M. Soil texture and plant degradation predictive model (STPDPM) in national parks using artificial neural network (ANN). Model Earth Syst Environ. 2020;6(2):715-29. https://doi.org/10.1007/s40808-020-00723-y.
-
27.
Shams SR, Jahani A, Moeinaddini M, Khorasani N. Air carbon monoxide forecasting using an artificial neural network in comparison with multiple regression. Model Earth Syst Environ. 2020;6(3):1467-75. https://doi.org/10.1007/s40808-020-00762-5.
-
28.
Dasenaki ME, Thomaidis NS. Quality and Authenticity Control of Fruit Juices-A Review. Molecules. 2019;24(6):1014. [PubMed ID: 30871258]. [PubMed Central ID: PMC6470824]. https://doi.org/10.3390/molecules24061014.
-
29.
Jahani R, Yazdanpanah H, van Ruth SM, Kobarfard F, Alewijn M, Mahboubi A, et al. Novel Application of Near-infrared Spectroscopy and Chemometrics Approach for Detection of Lime Juice Adulteration. Iran J Pharm Res. 2020;19(2):34-44. [PubMed ID: 33224209]. [PubMed Central ID: PMC7667562]. https://doi.org/10.22037/ijpr.2019.112328.13686.
-
30.
Borille BT, Marcelo MCA, Ortiz RS, Mariotti KC, Ferrao MF, Limberger RP. Near infrared spectroscopy combined with chemometrics for growth stage classification of cannabis cultivated in a greenhouse from seized seeds. Spectrochim Acta A Mol Biomol Spectrosc. 2017;173:318-23. [PubMed ID: 27673500]. https://doi.org/10.1016/j.saa.2016.09.040.
-
31.
Shojaee AliAbadi MH, Karami-Osboo R, Kobarfard F, Jahani R, Nabi M, Yazdanpanah H, et al. Detection of lime juice adulteration by simultaneous determination of main organic acids using liquid chromatography-tandem mass spectrometry. J Food Compost Anal. 2022;105:104223. https://doi.org/10.1016/j.jfca.2021.104223.
-
32.
Rinnan Å. Pre-processing in vibrational spectroscopy – when, why and how. Anal Methods. 2014;6(18):7124-9. https://doi.org/10.1039/c3ay42270d.
-
33.
Jolliffe IT, Cadima J. Principal component analysis: a review and recent developments. Philos Trans A Math Phys Eng Sci. 2016;374(2065):20150202. [PubMed ID: 26953178]. [PubMed Central ID: PMC4792409]. https://doi.org/10.1098/rsta.2015.0202.
-
34.
Brereton RG, Lloyd GR. Partial least squares discriminant analysis: taking the magic away. J Chemom. 2014;28(4):213-25. https://doi.org/10.1002/cem.2609.
-
35.
Galvao RK, Araujo MC, Jose GE, Pontes MJ, Silva EC, Saldanha TC. A method for calibration and validation subset partitioning. Talanta. 2005;67(4):736-40. [PubMed ID: 18970233]. https://doi.org/10.1016/j.talanta.2005.03.025.
-
36.
Flight L, Julious SA. The disagreeable behaviour of the kappa statistic. Pharm Stat. 2015;14(1):74-8. [PubMed ID: 25470361]. https://doi.org/10.1002/pst.1659.
-
37.
Tharwat A. Classification assessment methods. Appl Comput Inform. 2020;17(1):168-92. https://doi.org/10.1016/j.aci.2018.08.003.
-
38.
Li P, Li S, Du G, Jiang L, Liu X, Ding S, et al. A simple and nondestructive approach for the analysis of soluble solid content in citrus by using portable visible to near-infrared spectroscopy. Food Sci Nutr. 2020;8(5):2543-52. [PubMed ID: 32405410]. [PubMed Central ID: PMC7215219]. https://doi.org/10.1002/fsn3.1550.
-
39.
Yang Y, Zhang L, Hettinga KA, Erasmus SW, van Ruth SM. Prevalence of Milk Fraud in the Chinese Market and its Relationship with Fraud Vulnerabilities in the Chain. Foods. 2020;9(6):709. [PubMed ID: 32492929]. [PubMed Central ID: PMC7353633]. https://doi.org/10.3390/foods9060709.
-
40.
AIJN - European Fruit Juice Association. The AIJN Code of Practice. Brussel: AIJN - European Fruit Juice Association; 2016, [cited 2022]. Available from: https://aijn.eu/en/the-aijn-code-of-practice.
-
41.
Chen JY, Zhang H, Matsunaga R. Rapid determination of the main organic acid composition of raw Japanese apricot fruit juices using near-infrared spectroscopy. J Agric Food Chem. 2006;54(26):9652-7. [PubMed ID: 17177483]. https://doi.org/10.1021/jf061461s.
-
42.
Bai W, Yoshimura N, Takayanagi M. Quantitative Analysis of Ingredients of Blueberry Fruits by near Infrared Spectroscopy. J Near Infrared Spectrosc. 2014;22(5):357-65. https://doi.org/10.1255/jnirs.1129.
-
43.
Xie L, Ye X, Liu D, Ying Y. Prediction of titratable acidity, malic acid, and citric acid in bayberry fruit by near-infrared spectroscopy. Food Res Int. 2011;44(7):2198-204. https://doi.org/10.1016/j.foodres.2010.11.024.
-
44.
Grabska J, Ishigaki M, Bec KB, Wojcik MJ, Ozaki Y. Correlations between Structure and Near-Infrared Spectra of Saturated and Unsaturated Carboxylic Acids. Insight from Anharmonic Density Functional Theory Calculations. J Phys Chem A. 2017;121(18):3437-51. [PubMed ID: 28414469]. https://doi.org/10.1021/acs.jpca.7b02053.
-
45.
Weyer LG, Lo SC. Spectra- Structure Correlations in the Near-Infrared. Handbook of Vibrational Spectroscopy. New York: John Wiley & Sons; 2006. p. 1817-37. https://doi.org/10.1002/0470027320.s4102.
-
46.
Leon L, Kelly JD, Downey G. Detection of apple juice adulteration using near-infrared transflectance spectroscopy. Appl Spectrosc. 2005;59(5):593-9. [PubMed ID: 15969804]. https://doi.org/10.1366/0003702053945921.
-
47.
Liu F, He Y, Wang L, Sun G. Detection of Organic Acids and pH of Fruit Vinegars Using Near-Infrared Spectroscopy and Multivariate Calibration. Food Bioproc Tech. 2011;4(8):1331-40. https://doi.org/10.1007/s11947-009-0240-9.
-
48.
Subedi PP, Walsh KB, Hopkins DW. Assessment of Titratable Acidity in Fruit Using Short Wave near Infrared Spectroscopy. Part B: Intact Fruit Studies. J Near Infrared Spectrosc. 2012;20(4):459-63. https://doi.org/10.1255/jnirs.1011.
-
49.
Rodriguez N, Ortiz MC, Sarabia L, Gredilla E. Analysis of protein chromatographic profiles joint to partial least squares to detect adulterations in milk mixtures and cheeses. Talanta. 2010;81(1-2):255-64. [PubMed ID: 20188918]. https://doi.org/10.1016/j.talanta.2009.11.067.
-
50.
Fearn T, Riccioli C, Garrido-Varo A, Guerrero-Ginel JE. On the geometry of SNV and MSC. Chemometr Intell Lab Syst. 2009;96(1):22-6. https://doi.org/10.1016/j.chemolab.2008.11.006.
-
51.
Wilde AS, Haughey SA, Galvin-King P, Elliott CT. The feasibility of applying NIR and FT-IR fingerprinting to detect adulteration in black pepper. Food Control. 2019;100:1-7. https://doi.org/10.1016/j.foodcont.2018.12.039.
-
52.
Oliveri P. Class-modelling in food analytical chemistry: Development, sampling, optimisation and validation issues - A tutorial. Anal Chim Acta. 2017;982:9-19. [PubMed ID: 28734370]. https://doi.org/10.1016/j.aca.2017.05.013.
-
53.
Rodionova OY, Titova AV, Pomerantsev AL. Discriminant analysis is an inappropriate method of authentication. Trends Analyt Chem. 2016;78:17-22. https://doi.org/10.1016/j.trac.2016.01.010.
-
54.
Oliveri P, Malegori C, Mustorgi E, Casale M. Qualitative pattern recognition in chemistry: Theoretical background and practical guidelines. Microchem J. 2021;162:105725. https://doi.org/10.1016/j.microc.2020.105725.
-
55.
Gondim CS, Junqueira RG, Souza SVC, Ruisanchez I, Callao MP. Detection of several common adulterants in raw milk by MID-infrared spectroscopy and one-class and multi-class multivariate strategies. Food Chem. 2017;230:68-75. [PubMed ID: 28407966]. https://doi.org/10.1016/j.foodchem.2017.03.022.

