Abstract
Background:
Glutamine (Gln), as a precursor of glutathione and attenuation of pro-inflammatory cytokines, has a vital role in the antioxidant and anti-inflammatory defense of the body. Oxidative stress and inflammatory cytokines increase in respiratory diseases.Objectives:
We sought to investigate the effect of Gln supplementation on serum levels of some inflammatory and oxidative stress indices in hospitalized children with ARI.Methods:
We conducted a 5-day parallel-group, randomized controlled trial. This clinical trial was held for 5 days to assess the efficacy of the 0.5 g/kg body weight Gln, along with medical therapy, in hospitalized children with ARI.Results:
The difference in the high-sensitivity C-reactive protein (hs-CRP) between the Gln and placebo groups was significant after the intervention (analyzed by analysis of covariance [ANCOVA] after adjusting for the duration of cough and biochemical baseline values, 10.67 [7.77] vs 14.04 [6.57], respectively; P = 0.005). Moreover, at the end of the trial, there was no significant difference regarding the duration of hospitalization between the Gln and placebo groups (3.25 [1.37] vs 3.35 [0.8], respectively; P = 0.70).Conclusions:
The effect of Gln supplementation on the reduction of hs-CRP in children with ARI was demonstrated in this study. Further research is needed to determine the exact effect of Gln on inflammatory and oxidative stress biomarkers in children with ARI.Keywords
Acute Respiratory Infection Children Glutamine Cytokines Oxidative Stress
1. Background
Acute respiratory infection (ARI) is one of the most deadly infectious diseases in children worldwide (in both developed and developing countries), causing discomfort, frequent healthcare visits, and deaths (1). It is estimated that between 1.6 and 2.2 million children die each year from ARI (2). The mortality of ARI varies in different regions of the world (3) and accounts for up to 50% of visits of children to health facilities globally (4). Several socio-cultural, demographic, and environmental risk factors, such as female sex, age, comorbid diseases, nutrition, low-income status, maternal lower age, maternal lower education, place of residence (urban or rural), and wet season, predispose children younger than 5 years to ARI (5). Approaches to control ARIs according to the pneumonia severity usually include 4 basic classes: immunization against specific pathogens, early detection, and therapy of disease, enhancements in nutrition, and appropriate environments (6). Assessments of the World Health Organization (WHO) indicate that improvements in nutrition may decrease the risk of ARI incidence or mortality in children of developing countries (7). Therefore, any nutritional interventions can ameliorate child survival from infectious respiratory.
Glutamine (Gln) is the most plentiful free amino acid in plasma and tissue, synthesized in the lungs, liver, brain, skeletal muscles, and adipose tissue and secreted into the circulation (8). Most consumers of Gln are the small intestine, leukocytes, liver, and kidneys (9). Gln has important and regulatory functions in metabolism (as the lipogenic and glucogenic precursor and oxidative energy), protein synthesis and degradation, cell survival and growth, and expression of genes associated with metabolism. Moreover, Gln, as a precursor of glutathione and attenuation of pro-inflammatory cytokines, has a vital role in the antioxidant and anti-inflammatory defense of the body (10-14). As a conditionally essential amino acid, Gln is an essential element in the proliferation and function of immune cells; hence, Gln deficiency may have an intense impact on the immune system and may elevate the risk of respiratory infections. In catabolic conditions, Gln levels fall below normal, mainly in the muscle and liver (15, 16). Endogenous Gln synthesis does not provide the human body’s needs in catabolic conditions, including severe and long-term physical exercise, trauma, cancer, surgeries, sepsis, and infections. Under physiological conditions, Gln is efficiently synthesized in the liver and skeletal system. However, under catabolic situations and oxidative stress, concentrations of Gln in tissues decrease swiftly to assist the further demands of the body, resulting in energy metabolism disruptions and a weakened immune system (16). These disturbances can be lessened by supplementation with Gln; accordingly, it is currently a component of clinical nutrition supplementation practices and/or introduced for patients with immune suppression (17).
Given the increase of oxidative stress (18-20) and inflammatory cytokines in respiratory diseases (21, 22) and the high prevalence of ARI in children, we sought for the first time to investigate the effects of Gln supplementation on serum levels of tumor necrosis factor α (TNF-α), interleukin 1 beta (IL-1β), high-sensitivity C-reactive protein (hs-CRP), malondialdehyde (MDA), and total antioxidant capacity (TAC) in hospitalized children with ARI.
2. Methods
2.1. Study Design
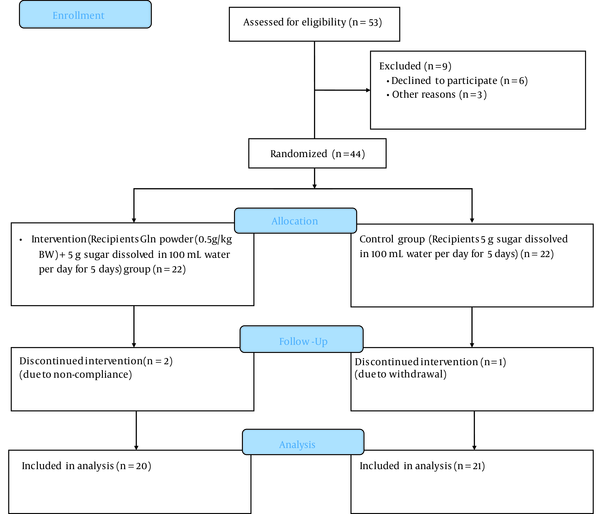
We conducted a 5-day parallel-group, randomized controlled trial. This clinical trial was carried out at the Bu Ali Hospital, Ardabil University of Medical Sciences, for 5 days to determine the efficacy of the 0.5 g/kg body weight Gln, along with medical therapy, in hospitalized children with ARI (Figure 1).
Consolidated Standards of Reporting Trials 2010 flow diagram
2.2. Ethics and Trial Registration
The study protocol was approved by the Ethics Committee of Ardabil University of Medical Sciences (code: IR.ARUMS.REC.1398.122), and it was conducted in accordance with the Helsinki Declaration. This clinical trial was registered on the Iranian Registry of Clinical Trials website (registration number: IRCT20210913052455N1).
2.3. Participants and Intervention
By referring to the Bu Ali Hospital, Ardabil, Iran, 44 children with ARI who met the inclusion criteria were recruited in this trial, and written informed consent was obtained from their parents (May 2020 to July 2020). Inclusion criteria were age between 2 to 6 years, having ARI, and not having any chronic disease such as congenital heart diseases, chronic liver or renal diseases, immune deficiency, or malignancy. Exclusion criteria were refusal to continue and consumption of less than 10% of total administered powders. The diagnosis of ARI and the general management of ARI were made by pediatric physicians. The sample size was determined based on serum MDA as the primary outcome obtained from our previous study (23). The sample size was computed using the following formula:
2.4. Assessment of Anthropometric Parameters
A trained dietitian measured anthropometric variables, including body weight, height, and body mass index (BMI), after overnight fasting (27). Body weight was measured by a Seca scale with an accuracy of 100 g at the beginning and end of the trial. Height was assessed in a relaxed position using a Seca portable stadiometer with an accuracy of 0.5 cm. BMI was calculated as body weight in kilograms divided by the square of height in meters at baseline and the end of the study (28).
2.5. Assessment of Appetite
Appetite was evaluated by the Council on Nutrition Appetite Questionnaire (CNAQ), which was adapted by Wilson et al. (29). CNAQ contains 8 items, and the scales of each item range from 1 to 5. Thus, the range of the total score is 8 to 40 points. A score of less than 28 is a cause for concern. The validity of CNAQ has been confirmed in the Iranian population (Cronbach α = 0.77) (30).
2.6. Assessment of Biochemistry Variables
Before and after the trial, venous blood samples (2 mL) were collected after 10-12 hours of overnight fasting. Enzyme-linked immunosorbent assay (ELISA) kits (Zell Bio GmbH) were used to determine serum levels of IL-1β, TNF-α, MDA, and TAC. Moreover, an ELISA kit (Pars Azmoun Co, Karaj, Iran) was applied to determine serum levels of hs-CRP (31).
2.7. Statistical Analysis
The data analyst was blinded after assignment to interventions. Statistical analysis was performed using SPSS version 23 (SPSS Inc, Chicago, Ill, USA). P values less than 0.05 were considered statistically significant. The Kolmogorov-Smirnov test was used to assess the normal distribution of the data. A chi-square test was applied to compare the categorical variables between the 2 groups at the baseline. The independent samples t test and Mann-Whitney U test were used to compare differences in parametric continuous and nonparametric data between the 2 groups, respectively. To compare within-group changes in variables, the paired-samples t test or Wilcoxon signed-rank test were used. To control confounding variables (TNF-α, IL-1β, hs-CRP, MDA, and TAC baseline values), analysis of covariance (ANCOVA) was applied to identify any differences between the groups after the intervention, adjusting for baseline values and covariates.
3. Results
Two patients in the Gln group and 1 patient in the placebo group were lost to follow-up due to non-compliance and withdrawal, respectively. The Gln and placebo groups were homogenous regarding the economic status, gender, place of residence, fever at the beginning, dyspnea age, height, weight, BMI, and appetite (P > 0.05; Table 1). However, the study groups had a significant difference in days of cough (2.45 [0.76] vs 1.8 [0.61]; P = 0.005).
| Variables | Gln Group (n = 20) | Placebo Group (n = 21) | Statistical Indicators |
|---|---|---|---|
| Economic status b | 0.629 | ||
| Equal income and expense | 8 (40.0) | 6 (30.0) | |
| Income more than expense | 7 (35.0) | 10 (50.0) | |
| Income less than expense | 5 (25.0) | 4 (20.0) | |
| Gender b | 0.501 | ||
| Girl | 15 (75.0) | 12 (60.0) | |
| Boy | 5 (25.0) | 8 (40.0) | |
| Fever at the beginning b | 0.366 | ||
| Yes | 15 (75.0) | 13 (65.0) | |
| No | 5 (25.0) | 7 (35.0) | |
| Dyspnea b | 0.698 | ||
| Yes | 18 (90.0) | 18 (90.0) | |
| No | 2 (10.0) | 2 (10.0) | |
| Age c | 4 (2.25 - 4.75) | 3.75 (2.25 - 4) | 0. 741 |
| Weight (kg) d | 15.36 (3.12) | 15.15 (2.63) | 0.817 |
| Height (cm) c | 98.2 (7.61) | 98.88 (6.96) | 0.796 |
| BMI (kg/m2) c | 15.49 (2.01) | 15.23 (1.74) | 0.660 |
| Duration of cough (day) d | 2.50 (2 - 3) | 2 (1 - 2) | 0.005 |
| Appetite c | 23.2 (9.09) | 18.9 (5.95) | 0.086 |
As presented in Table 2, there were no significant differences between the Gln and placebo groups in terms of biochemical measurements (P > 0.05) at the baseline, except for hs-CRP (P = 0.004). At the end of the intervention, the serum values of IL-1β, TNF-α, and hs-CRP significantly decreased in the Gln group (P < 0.05). In the placebo group, there were no significant changes in serum levels of biochemical variables at the end of the trial compared to baseline (P < 0.05). The difference in hs-CRP between the Gln and placebo groups after 5 days of intervention was significant (analyzed by ANCOVA after adjusting for the duration of cough and biochemical baseline values). Moreover, at the end of the study, there was no significant difference regarding the duration of hospitalization between the Gln and placebo groups (3.25 [1.37] vs 3.35 [0.8], respectively; P = 0.70).
Biochemical Parameter Values of the Study Groups at Baseline and at the End of the Intervention
| Variable | Gln Group (n = 20) | Placebo Group (n = 21) | P Value |
|---|---|---|---|
| IL-1β (mIU/mL) a | |||
| Before | 2917.18 (2403.70) | 4206.25 (2989.75) | 0.183 b |
| After | 2000.11 (1493.45) | 2934.29 (2190.28) | 0.391c |
| MD, pd | -917.05, 0.028 e | -1271.96, 0.117 | |
| TNF-α (ng/L) f | |||
| Before | 433.2 (231.10 - 924.65) | 358.1 (152.3 - 873.52) | 0.277b |
| After | 216.8 (143.60 - 503.52) | 219.20 (175.77 - 649.50) | 0.798c |
| MD, pd | -239.22, 0.002 e | -110.17, 0.167 | |
| hs-CRP (mg/L) | |||
| Before | 18.37 (7.02) | 11.53 (7.11) | 0.004b,e |
| After | 10.67 (7.77) | 14.04 (6.57) | 0.005c,e |
| MD, pd | -7.69, < 0.001 e | -2.51, 0.341 | |
| MDA (nmol/mL) | |||
| Before | 2.08 (0.70) | 1.99 (0.63) | 0.779b |
| After | 1.84 (0.35) | 1.81(0.25) | 0.930c |
| MD, pd | -0.24, 0.136 | -0.122, 0.465 | |
| TAC (nmol/mL) f | |||
| Before | 0.85 (0.74 - 1.07) | 0.84 (0.73 - 1.17) | 0.373b |
| After | 0.86 (0.74 - 1.10) | 1.1 (0.96 - 1.37) | 0.100c |
| MD, pd | 0.06, 0.395 | 0.185, 0.769 |
4. Discussion
According to the best of our knowledge, no study has examined the effects of Gln supplementation, along with medical therapy, in hospitalized children with ARI. It was shown that in the intervention group, hs-CRP was significantly reduced compared to the placebo group. Higher hs-CRP levels were significantly associated with poor respiratory function in children (32). In ARI, periodic assessment of CRP can instruct the true recovery or deteriorating phases of infection independently from viable signs and symptoms (33). In a recent study, reduced immunoglobulin A and CD4+ CD25+ T cells percentage, as well as elevated hs-CRP, IL-10, and procalcitonin, were related to pneumonia in children with COVID-19 (34). Abdollahi et al. concluded that hs-CRP measurement could be effective in the prediction of early neonatal sepsis (35). Moreover, according to the findings of studies by Xia et al. (36) and Lu et al. (37), the detection of hs-CRP or CRP is helpful for differential diagnosis and estimating the therapeutic effect of ARI in children. Since the elevation of hs-CRP and other inflammatory biomarkers is related to pneumonia in children, a decrease of inflammatory biomarkers through safe supplements such as Gln, along with medicine, can help to cure ARI and reduce infectious morbidity rates.
In the randomized controlled trial by Cai et al., Gln supplementation and recombinant human growth hormone for 14 days induced a significant reduction in CRP in critically ill elderly patients (38). In another study, parenteral Gln decreased CRP levels and infectious morbidity rates in patients with severe acute pancreatitis (39). Moreover, the combination of normal saline, hydroxyethyl starch, and Gln in severe acute pancreatitis resulted in a reduction in serum TNF-α, IL-8, and CRP concentrations (40). In the study by Wischmeyer et al. parenteral Gln administration in severe burn patients for 14 days reduced CRP in the Gln group, although this reduction was not statistically significant compared with the control group (41). We failed to show any significant reduction in other inflammatory indices compared to the placebo group. However, in the study by Ameho et al., Gln contributed to reducing the concentrations of the pro-inflammatory biomarkers, including IL-8 and TNF-α, in inflamed colonic tissues, leading to disease amelioration in experimental trinitrobenzene sulfuric acid-induced colitis in rats (42). Various studies have revealed that nutrition support supplemented with specific immunonutrients such as Gln may improve intestinal integrity and modulate acute phase responses (43-45), but the precise mechanism of Gln’s protection is unknown.
In the present study, the Gln administration did not change MDA and TAC levels. In line with our findings, enteral Gln administration of 45 g/d for 5 days did not change serum levels of MDA compared to the control group in patients with peritonitis or abdominal trauma (24). Seven days of Gln supplementation (0.15 g/kg) did not improve oxidative stress indices (MDA and TAC) in young, healthy men (46). Gln supplementation for 5 days increased the levels of 70-kd heat-shock protein as an oxidative stress marker and did not affect IL-10 and IL-6 levels in critically ill children (26). We presume that the differences between the conducted studies may be related to various patient groups, doses, and durations of Gln administration. The strengths of our study were the use of a placebo, regular follow-up of participants for supplement consumption, and evaluation of the effect of Gln on inflammatory and oxidative stress biomarkers levels in ARI for the first time. Limitations of this trial were lack of nutritional intake evaluation, short duration of Gln supplementation, and small sample size.
4.1. Conclusions
The effect of Gln supplementation on the reduction of hs-CRP in children with ARI was demonstrated in this study. Further studies are needed to determine the exact effects of Gln on inflammatory and oxidative stress biomarkers in children with ARI.
Acknowledgements
References
-
1.
Rudasingwa G. Potential risk factors contributing to acute respiratory infections among under 5 years children in Rwanda. Int J Infect Dis. 2020;101. https://doi.org/10.1016/j.ijid.2020.09.831.
-
2.
Williams BG, Gouws E, Boschi-Pinto C, Bryce J, Dye C. Estimates of world-wide distribution of child deaths from acute respiratory infections. Lancet Infect Dis. 2002;2(1):25-32. [PubMed ID: 11892493]. https://doi.org/10.1016/s1473-3099(01)00170-0.
-
3.
G. B. D. Child Mortality Collaborators. Global, regional, national, and selected subnational levels of stillbirths, neonatal, infant, and under-5 mortality, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1725-74. [PubMed ID: 27733285]. [PubMed Central ID: PMC5224696]. https://doi.org/10.1016/S0140-6736(16)31575-6.
-
4.
West TE, Goetghebuer T, Milligan P, Mulholland EK, Weber MW. Long-term morbidity and mortality following hypoxaemic lower respiratory tract infection in Gambian children. Bull World Health Organ. 1999;77(2):144-8. [PubMed ID: 10083713]. [PubMed Central ID: PMC2557604].
-
5.
Dagne H, Andualem Z, Dagnew B, Taddese AA. Acute respiratory infection and its associated factors among children under-five years attending pediatrics ward at University of Gondar Comprehensive Specialized Hospital, Northwest Ethiopia: institution-based cross-sectional study. BMC Pediatr. 2020;20(1):93. [PubMed ID: 32111196]. [PubMed Central ID: PMC7047350]. https://doi.org/10.1186/s12887-020-1997-2.
-
6.
Simoes EA, Cherian T, Chow J, Shahid-Salles SA, Laxminarayan R, John TJ. Acute respiratory infections in children. In: Jamison DT, Breman JG, Measham AR, Alleyne G, Claeson M, Evans DB, et al., editors. Disease Control Priorities in Developing Countries. 2nd ed. New York: Oxford University Press; 2006.
-
7.
Roth DE, Caulfield LE, Ezzati M, Black RE. Acute lower respiratory infections in childhood: opportunities for reducing the global burden through nutritional interventions. Bull World Health Organ. 2008;86(5):356-64. [PubMed ID: 18545738]. [PubMed Central ID: PMC2647440]. https://doi.org/10.2471/blt.07.049114.
-
8.
Dechelotte P, Hasselmann M, Cynober L, Allaouchiche B, Coeffier M, Hecketsweiler B, et al. L-alanyl-L-glutamine dipeptide-supplemented total parenteral nutrition reduces infectious complications and glucose intolerance in critically ill patients: the French controlled, randomized, double-blind, multicenter study. Crit Care Med. 2006;34(3):598-604. [PubMed ID: 16505644]. https://doi.org/10.1097/01.CCM.0000201004.30750.D1.
-
9.
Calder PC, Newsholme P. Glutamine and the immune system. Nutrition and immune function. CABI; 2002. p. 109-32.
-
10.
Sifa D, Bai X, Zhang D, Hu H, Wu X, Wen A, et al. Dietary glutamine improves meat quality, skeletal muscle antioxidant capacity and glutamine metabolism in broilers under acute heat stress. J Appl Anim Res. 2018;46(1):1412-7. https://doi.org/10.1080/09712119.2018.1520113.
-
11.
Wischmeyer PE, Kahana M, Wolfson R, Ren H, Musch MM, Chang EB. Glutamine reduces cytokine release, organ damage, and mortality in a rat model of endotoxemia. Shock. 2001;16(5):398-402. [PubMed ID: 11699081]. https://doi.org/10.1097/00024382-200116050-00014.
-
12.
Alipanah-Moghadam R, Molazadeh L, Jafari-Suha Z, Naghizadeh-Baghi A, Mohajeri M, Nemati A. Glutamine supplementation can reduce some atherosclerosis markers after exhaustive exercise in young healthy males. Nutrition. 2022;94:111506. [PubMed ID: 34844156]. https://doi.org/10.1016/j.nut.2021.111506.
-
13.
Ren W, Xia Y, Chen S, Wu G, Bazer FW, Zhou B, et al. Glutamine Metabolism in Macrophages: A Novel Target for Obesity/Type 2 Diabetes. Adv Nutr. 2019;10(2):321-30. [PubMed ID: 30753258]. [PubMed Central ID: PMC6416106]. https://doi.org/10.1093/advances/nmy084.
-
14.
Raizel R, Leite JS, Hypolito TM, Coqueiro AY, Newsholme P, Cruzat VF, et al. Determination of the anti-inflammatory and cytoprotective effects of l-glutamine and l-alanine, or dipeptide, supplementation in rats submitted to resistance exercise. Br J Nutr. 2016;116(3):470-9. [PubMed ID: 27215379]. https://doi.org/10.1017/S0007114516001999.
-
15.
Wernerman J. Role of glutamine supplementation in critically ill patients. Curr Opin Anaesthesiol. 2008;21(2):155-9. [PubMed ID: 18443481]. https://doi.org/10.1097/ACO.0b013e3282f54fd6.
-
16.
Engel JM, Pitz S, Muhling J, Menges T, Martens F, Kwapisz M, et al. Role of glutamine administration on T-cell derived inflammatory response after cardiopulmonary bypass. Clin Nutr. 2009;28(1):15-20. [PubMed ID: 18835506]. https://doi.org/10.1016/j.clnu.2008.08.007.
-
17.
Cruzat V, Macedo Rogero M, Noel Keane K, Curi R, Newsholme P. Glutamine: Metabolism and Immune Function, Supplementation and Clinical Translation. Nutrients. 2018;10(11). [PubMed ID: 30360490]. [PubMed Central ID: PMC6266414]. https://doi.org/10.3390/nu10111564.
-
18.
Chen AC, Burr L, McGuckin MA. Oxidative and endoplasmic reticulum stress in respiratory disease. Clin Transl Immunology. 2018;7(6). e1019. [PubMed ID: 29928501]. [PubMed Central ID: PMC5999202]. https://doi.org/10.1002/cti2.1019.
-
19.
Ozsurekci Y, Aykac K. Oxidative Stress Related Diseases in Newborns. Oxid Med Cell Longev. 2016;2016:2768365. [PubMed ID: 27403229]. [PubMed Central ID: PMC4926016]. https://doi.org/10.1155/2016/2768365.
-
20.
Bwititi P, Chinkwo K. Oxidative stress markers in infectious respiratory diseases: current clinical practice. Int J Res Med Sci. 2016;6(4):1802-13. https://doi.org/10.18203/2320-6012.ijrms20161727.
-
21.
Gan WQ, Man SF, Senthilselvan A, Sin DD. Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysis. Thorax. 2004;59(7):574-80. [PubMed ID: 15223864]. [PubMed Central ID: PMC1747070]. https://doi.org/10.1136/thx.2003.019588.
-
22.
Chung KF. Cytokines as targets in chronic obstructive pulmonary disease. Curr Drug Targets. 2006;7(6):675-81. [PubMed ID: 16787167]. https://doi.org/10.2174/138945006777435263.
-
23.
Nemati A, Alipanah-Moghadam R, Molazadeh L, Naghizadeh Baghi A. The Effect of Glutamine Supplementation on Oxidative Stress and Matrix Metalloproteinase 2 and 9 After Exhaustive Exercise. Drug Des Devel Ther. 2019;13:4215-23. [PubMed ID: 31849453]. [PubMed Central ID: PMC6912001]. https://doi.org/10.2147/DDDT.S218606.
-
24.
Kumar S, Kumar R, Sharma SB, Jain BK. Effect of oral glutamine administration on oxidative stress, morbidity and mortality in critically ill surgical patients. Indian J Gastroenterol. 2007;26(2):70-3. [PubMed ID: 17558069].
-
25.
Javanamani R, Nakhostin-Roohi B. [The Effect of One-week Glutamine Supplementation on Oxidative Stress Indices in Healthy Young Men]. J Ardabil Univ Med Sci. 2015;15(1):83-9. Persian.
-
26.
Jordan I, Balaguer M, Esteban ME, Cambra FJ, Felipe A, Hernandez L, et al. Glutamine effects on heat shock protein 70 and interleukines 6 and 10: Randomized trial of glutamine supplementation versus standard parenteral nutrition in critically ill children. Clin Nutr. 2016;35(1):34-40. [PubMed ID: 25701159]. https://doi.org/10.1016/j.clnu.2015.01.019.
-
27.
Aghamohammadi khiavi V, Pourghassem Gargari B, Aliasgharzadeh A. [Effect of Folic Acid Supplementation on Indices of Glycemic Control, Insulin Resistance and Lipid Profile in Patients With Type 2 Diabetes Mellitus]. Iran J Endocrinol Metab. 2011;13(4):354-60. Persian.
-
28.
Haidari F, Aghamohammadi V, Mohammadshahi M, Ahmadi-Angali K. Effect of whey protein supplementation on levels of endocannabinoids and some of metabolic risk factors in obese women on a weight-loss diet: a study protocol for a randomized controlled trial. Nutr J. 2017;16(1):70. [PubMed ID: 29061179]. [PubMed Central ID: PMC5654050]. https://doi.org/10.1186/s12937-017-0294-x.
-
29.
Wilson MM, Thomas DR, Rubenstein LZ, Chibnall JT, Anderson S, Baxi A, et al. Appetite assessment: simple appetite questionnaire predicts weight loss in community-dwelling adults and nursing home residents. Am J Clin Nutr. 2005;82(5):1074-81. [PubMed ID: 16280441]. https://doi.org/10.1093/ajcn/82.5.1074.
-
30.
Shafiei Sabet M. [Relationship Between Nutritional Status And Appetite Among Hiv Patients On Haart]. Nutr Food Sci Res. 2014;1(Suppl. (1)):236. Persian.
-
31.
Tamaddoni A, Nasseri E, Mohammadi E, Qujeq D, Zayeri F, Zand H, et al. A Double-blind Randomized Controlled Trial of Curcumin for Improvement in Glycemic Status, Lipid Profile and Systemic Inflammation in β-Thalassemia Major. J Herb Med. 2020;21. https://doi.org/10.1016/j.hermed.2019.100324.
-
32.
Soferman R, Glatstein M, Sivan Y, Weisman Y. HsCRP levels: measurement of airway inflammation in asthmatic children. Pediatr Int. 2008;50(1):12-6. [PubMed ID: 18279198]. https://doi.org/10.1111/j.1442-200X.2007.02517.x.
-
33.
Das CS. Acute Respiratory Ailments in Pediatric Age Group and Role of CRP in Diagnosis and Management. In: Ansar W, Ghosh S, editors. Clinical Significance of C-reactive Protein. Singapore: Springer; 2020. p. 213-48.
-
34.
Li Y, Deng W, Xiong H, Li H, Chen Z, Nie Y, et al. Immune-related factors associated with pneumonia in 127 children with coronavirus disease 2019 in Wuhan. Pediatr Pulmonol. 2020;55(9):2354-60. [PubMed ID: 32543756]. [PubMed Central ID: PMC7323435]. https://doi.org/10.1002/ppul.24907.
-
35.
Abdollahi A, Shoar S, Nayyeri F, Shariat M. Diagnostic Value of Simultaneous Measurement of Procalcitonin, Interleukin-6 and hs-CRP in Prediction of Early-Onset Neonatal Sepsis. Mediterr J Hematol Infect Dis. 2012;4(1). e2012028. [PubMed ID: 22708043]. [PubMed Central ID: PMC3375671]. https://doi.org/10.4084/MJHID.2012.028.
-
36.
Xia R, Guo F, Li Z, Wu R, Fang Q. Application of c-reactive protein test in children with acute respiratory infection [J]. Journal of Southeast University; 2004.
-
37.
Lu W, Li L, Chen Y, Ye J. Combined Detection of hs-CRP and WBC in Pediatric Infectious Diseases. Guide of China Medicine; 2013.
-
38.
Cai GL, Yan J, Yu YH, Zhang ZC, Gong SJ, Dai HW, et al. [Influence of glutamine and growth hormone intensified nutrition support on immunomodulation in critically ill elderly patients]. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2006;18(10):595-8. Chinese. [PubMed ID: 17038243].
-
39.
Fuentes-Orozco C, Cervantes-Guevara G, Mucino-Hernandez I, Lopez-Ortega A, Ambriz-Gonzalez G, Gutierrez-de-la-Rosa JL, et al. L-alanyl-L-glutamine-supplemented parenteral nutrition decreases infectious morbidity rate in patients with severe acute pancreatitis. JPEN J Parenter Enteral Nutr. 2008;32(4):403-11. [PubMed ID: 18596311]. https://doi.org/10.1177/0148607108319797.
-
40.
Zhao G, Zhang JG, Wu HS, Tao J, Qin Q, Deng SC, et al. Effects of different resuscitation fluid on severe acute pancreatitis. World J Gastroenterol. 2013;19(13):2044-52. [PubMed ID: 23599623]. [PubMed Central ID: PMC3623981]. https://doi.org/10.3748/wjg.v19.i13.2044.
-
41.
Wischmeyer PE, Lynch J, Liedel J, Wolfson R, Riehm J, Gottlieb L, et al. Glutamine administration reduces Gram-negative bacteremia in severely burned patients: a prospective, randomized, double-blind trial versus isonitrogenous control. Crit Care Med. 2001;29(11):2075-80. [PubMed ID: 11700398]. https://doi.org/10.1097/00003246-200111000-00006.
-
42.
Ameho CK, Adjei AA, Harrison EK, Takeshita K, Morioka T, Arakaki Y, et al. Prophylactic effect of dietary glutamine supplementation on interleukin 8 and tumour necrosis factor alpha production in trinitrobenzene sulphonic acid induced colitis. Gut. 1997;41(4):487-93. [PubMed ID: 9391247]. [PubMed Central ID: PMC1891521]. https://doi.org/10.1136/gut.41.4.487.
-
43.
Zhao G, Wang CY, Wang F, Xiong JX. Clinical study on nutrition support in patients with severe acute pancreatitis. World J Gastroenterol. 2003;9(9):2105-8. [PubMed ID: 12970916]. [PubMed Central ID: PMC4656684]. https://doi.org/10.3748/wjg.v9.i9.2105.
-
44.
Sahin H, Mercanligil SM, Inanc N, Ok E. Effects of glutamine-enriched total parenteral nutrition on acute pancreatitis. Eur J Clin Nutr. 2007;61(12):1429-34. [PubMed ID: 17311061]. https://doi.org/10.1038/sj.ejcn.1602664.
-
45.
Pearce CB, Sadek SA, Walters AM, Goggin PM, Somers SS, Toh SK, et al. A double-blind, randomised, controlled trial to study the effects of an enteral feed supplemented with glutamine, arginine, and omega-3 fatty acid in predicted acute severe pancreatitis. JOP. 2006;7(4):361-71. [PubMed ID: 16832133].
-
46.
Nakhostin-Roohi B, Javanamani R. The Effect of Glutamine Supplementation on Exercise-Induced Oxidative Stress. J Adv Agric Technol. 2015;2(1). https://doi.org/10.12720/joaat.2.1.8-12.