1. Background
Local anesthesia has gained more widespread acceptance than general anesthesia for intraocular surgery (1, 2). The mixture of lignocaine (2%) and bupivacaine (0.5% or 0.75%) is usually used to get the benefits of both drugs, including the long-lasting effect of bupivacaine and the rapid-onset action and greater motor block of lignocaine (3, 4).
Supplementary injections are sometimes used to establish more effective anesthesia. Hyaluronidase is one of the commonly used drugs in combination with local anesthesia in ophthalmic surgery (5, 6). It facilitates the spread of local anesthetics. Adrenaline is also a commonly used adjuvant to local anesthesia. It provides local hemostasis in the operative field and plays a role in delaying the absorption of local anesthetics. Such delay in absorption not only decreases the risk of systemic toxicity but also prolongs the duration of anesthesia (7).
The toxic effect of local anesthetics on the functional and morphologic integrity of the retina has been previously investigated in animal studies. Liang et al. (8) revealed that the intravitreal injection of a mixture of lidocaine and bupivacaine-induced neurophysiological changes in the rabbit retina. On the other hand, Zemel et al. (9) demonstrated that the dose of lidocaine required for retrobulbar anesthesia was not toxic to the rabbit retina.
Optical coherence tomography (OCT) is a noninvasive diagnostic technique that provides the in vivo measurement of retinal thickness. It uses near-infrared light to produce cross-sectional or 3D images of the retina. This technique utilizes a concept known as interferometry that creates a cross-sectional map of the retina (10). It allows for the examination of non-myelinated axons in the retinal nerve fiber layer (RNFL) (11), as well as ganglion cells in the macular area of the retina (12).
2. Objectives
The objective of this work was to study the effect of local anesthesia with lidocaine versus local anesthesia with lidocaine and an extra administration of adrenaline on the retinal layer thickness measured by OCT in patients indicated for elective cataract surgery.
3. Methods
3.1. Study Design and Participants
This is a randomized controlled trial conducted on 60 participants indicated for elective cataract surgery by phacoemulsification under local anesthesia with lidocaine. Participants were randomly assigned to one of the two groups. The first group received local anesthesia with lidocaine 2% with an extra administration of adrenaline (adrenaline group; n = 30 participants), and the second group received local anesthesia with lidocaine 2% only (control group; 30 participants). Randomization was done using a closed opaque envelope technique. The trial flow diagram is demonstrated in Figure 1. The patients were recruited from the Ophthalmology Outpatient clinics of Beni-Suef University Hospital between December 2017 and December 2018. The study adhered to the CONSORT guidelines. It was conducted following the Declaration of Helsinki and approved by the Faculty of Medicine, Beni-Suef University Research Ethics Committee (FMBSU-REC). The study was registered in the Pan African Clinical Trial Registry with identification number PACTR201907757137778.
The inclusion criteria included patients candidate for elective cataract surgery. However, the following patients were excluded from the study: patients with a history of hypertension or ischemic heart disease, patients with ocular comorbidities such as retinal pathology, reduced corneal sensation, ocular movement abnormality, nystagmus, ptosis, or facial nerve palsy, patients with any systemic disease known to affect the retina, patients receiving medications known to cause retinal toxicity, patients with allergy to local anesthetics, and patients refusing the local anesthetic technique.
The patients were subjected to the followings.
3.1.1. Optical Coherence Tomography
It was done for all the included patients preoperatively and one week postoperatively by an ophthalmologist who was blinded to the anesthetic technique using spectral-domain OCT (RTVUE XR, Optovue, USA). It was a swept-source OCT instrument with high axial resolution and low error in the measurement of retinal thickness. The OCT technique generates cross-sectional images of the retina with an axial resolution of 10.00 µm (13). The RNFL thickness scan protocol was applied (calculated by averaging three circumferential scans for 360° around the optic disc, 256 axial scans, and 3.4 µm diameter). If the participants’ pupils were not large enough to allow adequate OCT imaging, tropicamide 1% eye drops were used for pupillary dilatation. Internal fixation (blue light) was used for all OCT scans, and a patch was placed over the other eye to improve fixation. We recorded superior, inferior, and average RNFL thickness (averaging peripapillary retina for 360° around the optic disc).
3.1.2. Anesthetic Technique
All the included patients underwent elective cataract surgery under local anesthesia with lidocaine 2%. Thirty patients received local anesthesia with lidocaine 2%, and 30 patients received local anesthesia with lidocaine 2% and an extra administration of adrenaline (1:100,000). Hyaluronidase 90 IU was added to lidocaine before injection. Venous access was obtained in all patients preoperatively under complete antiseptic conditions. The patients were monitored for oxygen saturation, electrocardiogram, and noninvasive blood pressure. No premedication was given to the patients. We did not give sedation for the patients, and only psychological reassurance was done. In all included patients, a peribulbar injection was done under complete antiseptic conditions using a 25-mm 25-G needle. The inferolateral injection of 4 mL of the local anesthetic solution was done after negative aspiration while the patient was looking straight ahead. Then, 3 mL was injected through the medial canthus. We applied digital pressure for five seconds after each injection. The digital pressure was repeated every 20 seconds for about three minutes. Blood pressure was monitored for all included patients after injection.
3.2. Statistical Methods
The sample size was calculated based on a pretrial pilot study using G*Power version 3.1.9.2 software. To reach a statistical power (1-β) of 80%, a total of 60 participants were required. The probability of type I error (α) was 5%. The statistical analysis was done using the Statistical Package for Social Sciences version 18 (SPSS V 18). Descriptive data were expressed as mean ± SD for quantitative data and number (%) for categorical data. We used the independent samples’ t-test to compare the adrenaline and control groups for age and the chi-square test to compare the adrenaline and control groups for sex. We used the paired samples’ t-test to compare the pre and postoperative retinal thickness in both adrenaline and control groups. A mixed analysis of variance (ANOVA) test was used for comparing the adrenaline and control groups in the changes in the retinal thickness. The probability/significance value (P value) of < 0.05 was considered statistically significant.
4. Results
The mean age of patients in the adrenaline group (n = 30) was 57.07 ± 9.2 years, while the mean age of patients in the control group (n = 30) was 60.07 ± 6.33 years. Moreover, 53.3% (n = 16) of the patients in the adrenaline group were males, and 46.7% (n = 14) were females. In the control group, 33.3% (n = 10) were males and 66.7% (n = 20) were females. No significant difference was found between the groups in age (P value = 0.147) and sex (P value = 0.118) (Table 1).
| Adrenaline Group (N = 30) | Control Group (N = 30) | P Value | |
|---|---|---|---|
| Age in years | 57.07 ± 9.2 | 60.07 ± 6.33 | 0.147 |
| Sex | 0.118 | ||
| Male | 16 (53.3) | 10 (33.3) | |
| Female | 14 (46.7) | 20 (66.7) |
aValues are expressed as mean ± SD or No. (%).
bP value ≥ 0.05 (non-significant).
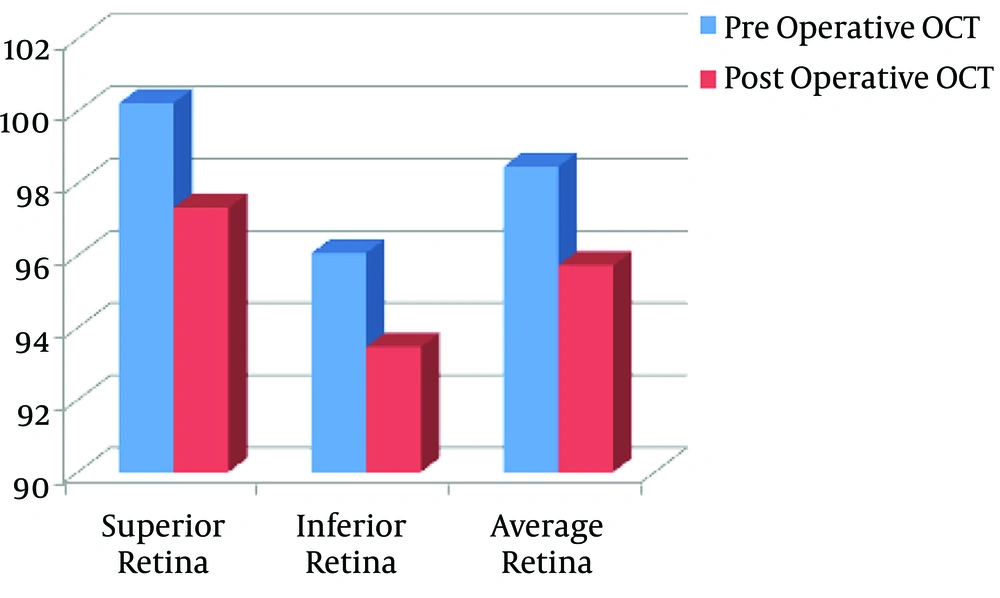
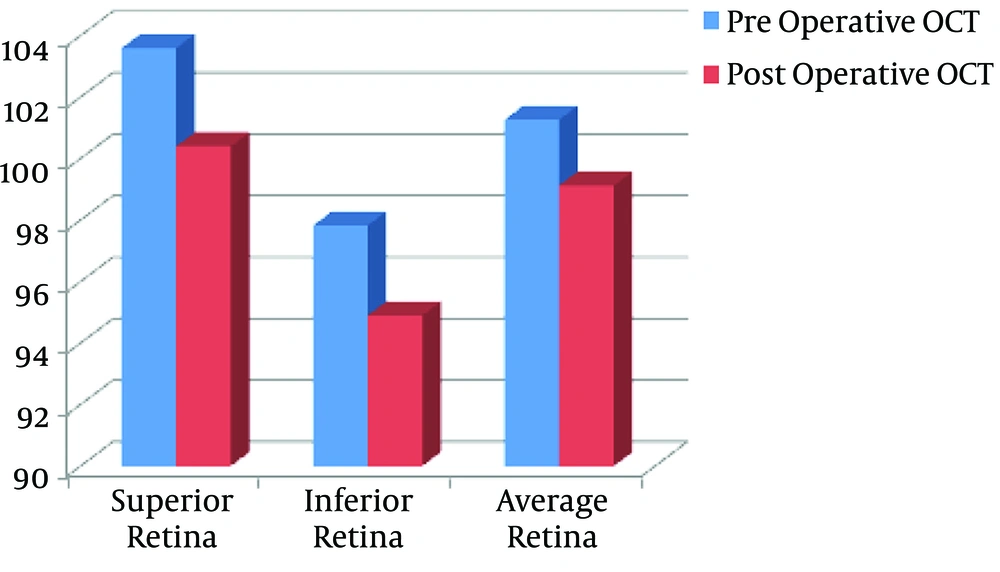
The OCT findings showed statistically significant decreases postoperatively in superior (P value = 0.028), inferior (P value = 0.017), and average (P value = 0.021) retinal thickness in the adrenaline group (Figure 2, Table 2). Moreover, there were statistically significant decreases postoperatively in superior (P value = 0.032), inferior (P value = 0.046), and average (P value = 0.028) retinal thickness in the control group (Figure 3, Table 2).
| Preoperative OCT | Postoperative OCT | P value | P value Between Groups | |
|---|---|---|---|---|
| Superior retina | 0.325 | |||
| Adrenaline group | 100.23 ± 14.61 | 97.33 ± 14.83 | 0.028b | |
| Control group | 103.6 ± 10.82 | 100.4 ± 11.61 | 0.032b | |
| Inferior retina | 0.642 | |||
| Adrenaline group | 96.07 ± 13.09 | 93.47 ± 14.4 | 0.017b | |
| Control group | 97.83 ± 13.9 | 94.9 ± 13.28 | 0.046b | |
| Average retina | 0.291 | |||
| Adrenaline group | 98.47 ± 11.7 | 95.73 ± 12.94 | 0.021b | |
| Control group | 101.27 ± 10.96 | 99.13 ± 10.67 | 0.028b |
aValues are expressed as mean ± SD.
bP value < 0.05 (significant).
Comparing the adrenaline and control groups for the OCT findings, there was no significant difference between the groups regarding decreases in superior (P value = 0.325), inferior (P value = 0.642), and average (P value = 0.291) retinal thickness (Table 2).
5. Discussion
The potential toxic effect of local anesthetics on retinal integrity has been previously investigated in animal studies but not in humans (8, 9). In this work, we aimed to study the effect of local anesthesia with lidocaine versus local anesthesia with lidocaine and an extra administration of adrenaline on the retinal layer thickness measured by OCT. Our study revealed that local anesthesia with lidocaine caused a significant decrease in postoperative superior, inferior, and average retinal thickness.
In line with our results, Grosskreutz et al. (13) revealed that lidocaine was toxic to retinal ganglion cells (RGCs) of rats both in vitro and in vivo. Similar findings were also reported by Stangos et al. (14) who studied the effect of intravitreal injection of lidocaine on the retina of rabbit eyes based on electroretinography (ERG) and histological examination of the retina. The ERG revealed the transient suppression of ERG b-wave for five to eight hours. Microscopically, there were some lesions in synapses between photoreceptors and both bipolar and horizontal cells (14). Additionally, intravitreal injection of lidocaine into the vitreous of cats’ eyes was found to be complicated by the occurrence of vacuolization in the retinal nerve fiber layer 24 h following injection (15).
In contrast to our findings, Zemel et al. (9) concluded that lidocaine and bupivacaine did not affect rabbit retinal function or structure at concentrations required for retrobulbar anesthesia. They assessed the retinal morphological structure by light microscopy and retinal function by electroretinography and visual evoked potential (9).
The significant toxic effect of lidocaine on the retina is mediated through the glutamate excitotoxic pathway (16). Lidocaine interferes with cytosolic calcium buffering and ATP-ase (17, 18), which make neurons liable to glutamate-mediated neurotoxicity. Although neurons are generally resistant to the toxicity of endogenous concentrations of glutamate, under special conditions of defective energy metabolism, these endogenous levels of glutamate become neurotoxic (19, 20).
Adrenaline is a commonly used adjunct to local anesthesia. It delays the absorption of the local anesthetic and prolongs and enhances its anesthetic effect. Such an effect is presumed to result from its vasoconstrictor effect that reduces local blood flow, thereby causing slower local anesthetic clearance from the injection site (7).
Our results revealed that the extra administration of adrenaline to lidocaine did not affect the post-anesthetic changes in the retinal thickness. This means that the potentiation of the anesthetic effect of lidocaine mediated by adrenaline was not sufficient to induce significant changes in the retinal thickness.
The main clinical implication of our work is the recommendation of using preoperative or intraoperative neuroprotective drugs to decrease the neurotoxic effect of anesthesia on the retina. Further research should be directed on a larger scale and for a longer duration to highlight if the effect of local anesthesia on the retinal thickness is reversible or not.
5.1. Conclusions
Local anesthesia with lidocaine in patients undergoing elective cataract surgery caused significant decreases in the retinal thickness (superior, inferior, and average). The extra administration of adrenaline to local anesthesia did not affect the post-anesthetic changes in the retinal thickness.