1. Context
Caesarean section is one of the major surgeries in women with an increasing trend in developed and developing countries (1-4). The rate of cesarean delivery in the United States and the European countries has been reported 32.7% and 21.1%, respectively (5). The rate of the cesarean section in Iran ranges from 26% to 66.5% according to various studies (6). The world’s report for the cesarean sections among 15 countries has been about 18.6% (7). Spinal anesthesia is the best-preferred method for anesthesia in patients with emergency and elective cesarean section due to a series of advantages such as rapid onset, high success rate, reduced side effects on the mother and the fetus, safety, and efficacy (8-11).
According to the Royal College of Obstetricians and Gynecologists, the cesarean section should be carried out under an urgent appropriate circumstance to eliminate the risks to the embryos and guarantee the mother’s safety (12). Any anesthetic drug or anesthetic method for the cesarean section should have features such as maintaining the hemodynamic stability of the mother, rapid induction and possessing the least effects on the newborn Apgar score (13). Cesarean section is of much sensitivity due to its adverse effects and complications on the mother and the baby. For example, moderate to severe pain after the cesarean section reported by 75% of the mothers is one of the major concerns of the mothers after the cesarean delivery (14).
Postoperative complications, such as shivering with a prevalence of about 56.7% and vomiting with 9 to 56% are the main concerns for anesthesiologists in the recovery room. Postoperative shivering can increase oxygen consumption and carbon dioxide production by up to 400%. On the other hand, nausea and vomiting can prolong the recovery (15-18). The neonatal Apgar score after birth is one of the determining factors of mortality and general wellbeing of the newborn. In this regard, the type of drug used for the cesarean section is very important in the Apgar score of the cesarean section (19).
Pethidine is an intermediate lipid-soluble opioid with both a postoperative analgesic mechanism and a sensory block which is known as the only substance used in the cesarean section under spinal anesthesia. However, pethidine can cause a number of complications such as hypotension, vomiting and respiratory depression (20, 21).
Considering the controversial results of various studies about different doses of intrathecal pethidine on the mother and the newborn this meta-analysis was conducted to determine the effect of minimum and maximum intrathecal doses of pethidine (5 to 40 mg) on the cesarean section under spinal anesthesia.
2. Evidence Acquisition
This is a meta-analysis and systematic review of the effects of intrathecal meperidine on maternal and newborn outcomes after cesarean section under spinal anesthesia that was carried out according to preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines (22).
2.1. Search Strategy
We performed a systemic search without any language restrictions from 1 September 2018 to 7 December 2018 (literature searches between 1990 to 2018). Our searching medical databases for this study included ISI, PubMed, Scopus, Google Scholar, Barakat, MagIran, SID, Irandoc, and EMBASE. The keywords used for medical subjects included meperidine, pethidine, meperidine hydrochloride, cesarean section, pethidine and cesarean, spinal anesthesia, Apgar score, intrathecal anesthesia, subarachnoid anesthesia, regional anesthesia, lumbar anesthesia, intrathecal meperidine, intrathecal pethidine, postoperative shivering, postoperative complication.
Two researchers at the same time conducted a study selection and data extraction of the effect of the minimum and maximum dose (5 to 40 mg) of intrathecal pethidine on maternal and newborn outcomes after cesarean section and independently evaluating the quality of the articles. Inclusion criteria were the use of the minimum and maximum dose of intrathecal pethidine (5 to 40 mg) in cesarean section under spinal anesthesia, matching of the drugs were used with pethidine, measuring one of the following parameters: shivering, nausea, vomiting, hypotension, pruritus, Apgar score, time of sensory block, time of motor block and analgesia duration. Exclusion criteria were the use of local anesthesia, non-elective surgery, combined different opioids, studies that were unrelated to the subject and studies that full text was unavailable.
2.2. PICO
Population included cesarean section under spinal anesthesia that received intrathecal meperidine. Patients were divided into pethidine and control groups. The outcome included: shivering, nausea, vomiting, hypotension, pruritus, Apgar score, time of sensory block, time of motor block, and analgesia duration.
2.3. Statistical Analysis
The following data were extracted from the studies: the authors' names, the year of publication, the type of study, the sample size of study groups, country of publication, prevalence of shivering, nausea, vomiting, hypotension, pruritus, and Apgar score at one and five minutes, time of sensory block, time of complete motor block, and analgesia duration.
Effect size parameters consisted of relative risk (RR) and standard mean difference (SMD) in this study. The results of studies pooled using random effects models and fixed effects models. Heterogeneity of studies checked using Q test, I2 index and meta-regression. Publication bias checked using funnel plot and Egger test. P value < 0.05 was considered statistically significant. Data were analyzed with STATA ver. 11.2.
3. Results
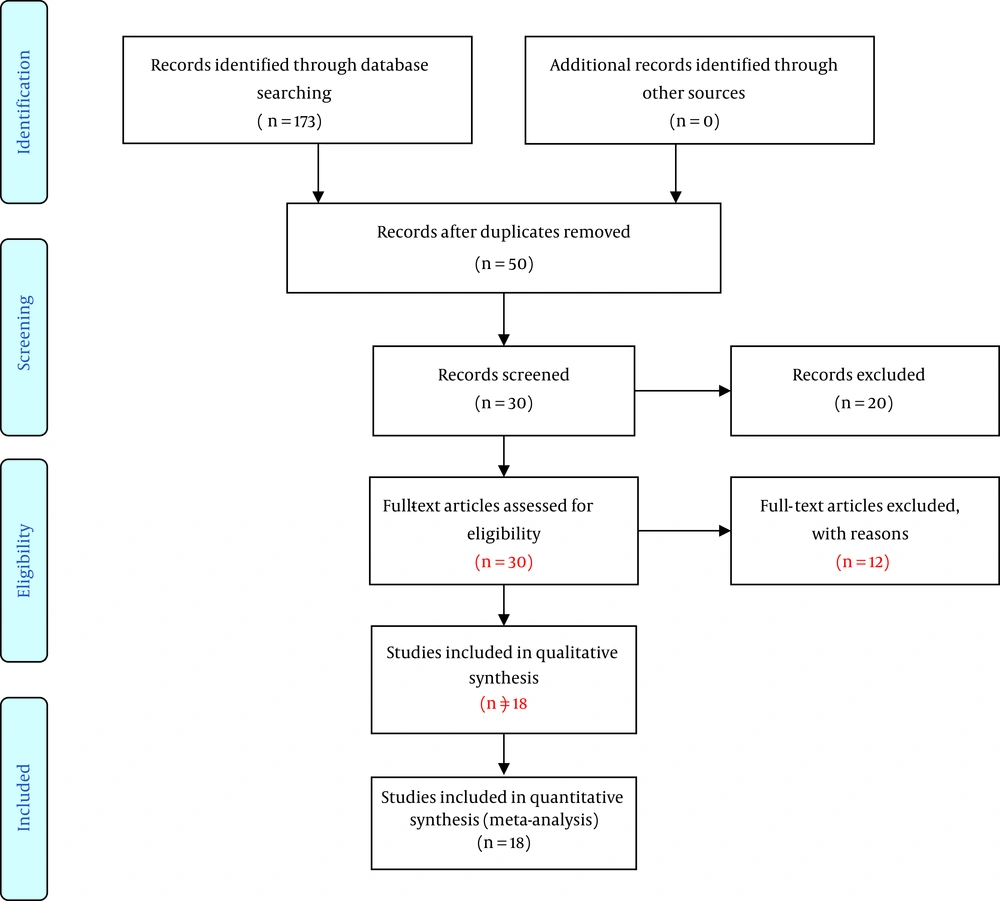
Selection of Articles: in this review, 50, 30, and 93 articles were obtained from PubMed, ISI, SCOPUS and other databases, respectively, according to the keywords. After excluding the duplicates, 50 articles remained. Looking at the summary of the articles, 20 articles that were not related to the topic were deleted as well. By reading the full text of the articles, 5 articles were eliminated because of the lack of sample size and 7 more others were excluded because of the unspecified drug usage. Finally, 18 articles were entered into the analysis (Figure 1).
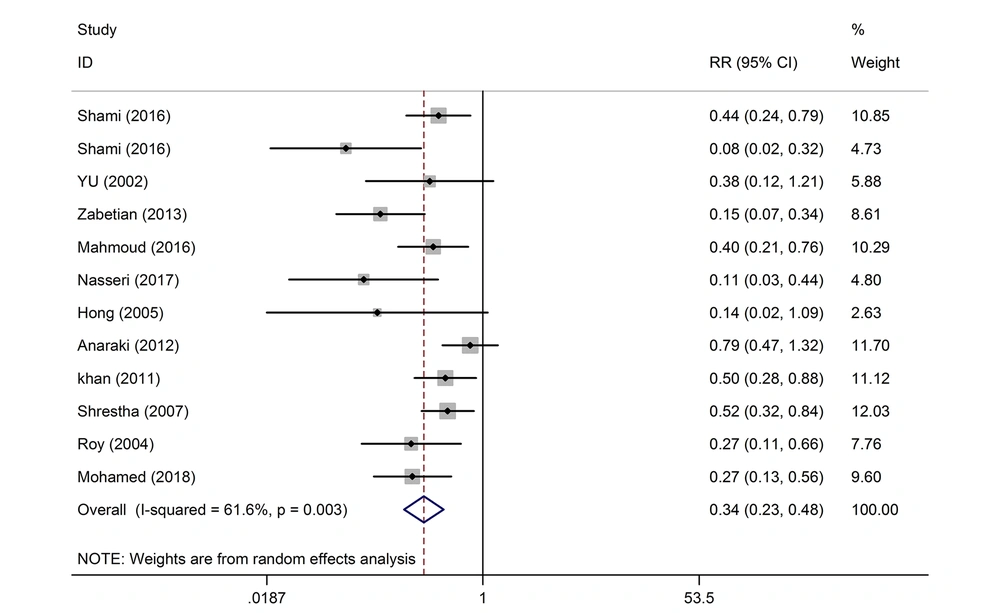
Eighteen articles that had examined factors such as nausea, vomiting, shivering, hypotension, pruritus, newborn Apgar score, duration of the postoperative analgesia, and duration of the complete sensory, and motor block are presented in Tables 1 and 2. The length of each segment shows the confidence interval. The middle of the line represents the relative risk for the point estimation. The Rhombus sign represents the combined result of all the studies. The reason for the cut-off point of 20 mg in this study was due to high and low effects of pethidine and statistically similar placement of studies with different doses on both sides of the cut-off point. The sample size of the studies was 1,494. Out of the 12 studies with a dose of ≤ 20 mg, 3 studies were not meaningful in terms of postoperative shivering. On the whole, the effect of the intrathecal pethidine with a dose of ≤ 20 mg on the postoperative shivering was significant. The weight of the Shrestha et al. (23) study was more. Those who had received pethidine had lower shivering of 66%. The studies with a greater confidence interval (CI) had less weight and were less precise. The effect of the intrathecal pethidine with a dose of > 20 mg on the postoperative shivering in 8 studies was significant except for one case. Patients who had received intrathecal pethidine at a dose of > 20 mg showed lower shivering of 82% (Table 3 and Figure 2).
| Authors’ Names | Year | Country | Dose, mg | Pethidine/Control (Number) | Variables | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Shivering | Nausea | Vomiting | Hypotension | Pruritus | Apgar > 7 at 1 min | Apgar > 7 at 5 min | |||||
| Shami et al. (10) | 2016 | Iran | 5 | Pethidine (n = 50) | 11 (22) | 25 (50) | 13 (26) | 33 (66) | 3 (6) | - | - |
| Control (n = 50) | 25 (50) | 19 (38) | 6 (12) | 34 (68) | 0.00 | - | - | ||||
| 10 | Pethidine (n = 50) | 2 (4) | 27 (54) | 13 (26) | 37 (74) | 13 (26) | - | - | |||
| Control (n = 50) | 25 (50) | 19 (38) | 6 (12) | 34 (68) | 0.00 | - | - | ||||
| Farzi et al. (11) | 2014 | Iran | 25 | Pethidine (n = 65) | - | 18 (27.6) | 6 (9.2) | 41 (63) | 0.00 | 59(90.7) | 65 (100) |
| Control (n = 65) | - | 35 (53.8) | 9 (13.8) | 35 (53.8) | 2 (3) | 62 (95.3) | 65 (100) | ||||
| Rastegarian (9) | 2013 | Iran | 25 | Pethidine (n = 50) | 4 (8) | 4 (8) | 5 (10) | 7 (14) | 0.00 | 44 (88) | 49 (98) |
| Control (n = 50) | 15 (30) | 0.00 | 0.00 | 6 (12) | 0.00 | 45 (90) | 49 (98) | ||||
| Atalay et al. (24) | 2010 | Turkey | 35 | Pethidine (n = 20) | 0.00 | 10 (50% | 5(25) | 11 (55) | 9 (45) | 17 (85) | 19 (95) |
| Control (n = 20) | 10 (50) | 10 (50) | 5(25) | 13 (65) | 0.00 | 18 (90) | 20 (100) | ||||
| 30 | Pethidine (n = 20) | 0.00 | 6 (30) | 3 (15) | 6 (30) | 7 (35) | 18 (90) | 20 (100) | |||
| Control (n = 20) | 10 (50) | 10 (50) | 5(25) | 13 (65) | 0.00 | 19 (95) | 20 (100) | ||||
| 25 | Pethidine (n = 20) | 0.00 | 4 (20) | 1 (5) | 4 (20) | 2 (10) | 17 (85) | 19 (95) | |||
| Control (n = 20) | 10 (50) | 10 (50) | 5 (25) | 13 (65) | 0.00 | 18 (90) | 19 (95) | ||||
| Yu et al. (21) | 2002 | China | 10 | Pethidine (n = 20) | 3 (15) | 11(55) | 11 (55) | 14 (70) | 5 (25) | - | - |
| Control (n = 20) | 8 (40) | 3 (15) | 3 (15) | 11 (55) | 0.00 | - | - | ||||
| Kouzegaran et al. (25) | 2018 | Iran | 5 | Pethidine (n = 20) | - | 16 (80) | 16 (80) | - | 3 (15) | - | - |
| Control (n = 20) | - | 16 (80) | 16 (80) | - | 0.00 | - | - | ||||
| Kusumasari et al. ((26) | 2013 | Indonesia | 10 | Pethidine (n = 98) | 35 (35.7) | 8 (8.1) | 8 (8.1) | 38 (38.7) | 0.00 | - | - |
| Control | - | - | - | - | - | - | - | ||||
| 20 | Pethidine (n = 98) | 22 (22.4) | 22 (22.4) | 22 (22.4) | 48 (48.9) | 0.00 | - | - | |||
| Control | - | - | - | - | - | - | - | ||||
| Hirmanpour et al. (27) | 2017 | Iran | 25 | Pethidine (n = 40) | 2 (5) | 28 (70) | 26 (65) | 13 (32.5) | 14 (35) | 36 (90) | 40 (100) |
| Control (n = 40) | 26 (65) | 22 (55) | 21 (52.5) | 9 (22.5) | 1 (2.5) | 37 (92.5) | 40 (100) | ||||
| Zebetian et al. (28) | 2013 | Iran | 10 | Pethidine (n = 35) | 5 (14.2) | - | - | 14 (40) | 0.00 | 29 (82.8) | 35 (100) |
| Control (n = 35) | 33 (94.2) | - | - | 16 (45.7) | 0.00 | 29 (82.8) | 35 (100) | ||||
| Mahmoud et al. (29) | 2016 | Egypt | 10 | Pethidine (n = 30) | 8 (26.6) | 7 (23.3) | 7 (23.3) | - | 2 (6.6) | 24 (80) | 30 (100) |
| Control (n = 30) | 20 (66.6) | 3 (10) | 3 (10) | - | 0.00 | 25 (83.3) | 29 (96.6) | ||||
| Nasseri et al. (30) | 2017 | Iran | 10 | Pethidine (n = 30) | 2 (6.6) | 12 (40) | 6 (20) | 20 (66.6) | 1 (3.3) | - | - |
| Control (n = 30) | 18 (60) | 10 (33.3) | 5 (16.6) | 21 (70) | 0.00 | - | - | ||||
| Kafle (31) | 1993 | Nepal | 10 | Pethidine (n = 25) | - | 2 (8) | 2 (8) | 8 (32) | 8 (32) | 19 (76) | 25 (100) |
| Control (n = 25) | 2 (8) | 2 (8) | 15 (60) | 0.00 | 19 (76) | 25 (100) | |||||
| Hong and Lee (32) | 2005 | South Korea | 10 | Pethidine (n = 30) | 1 (3.3) | 13 (43.3) | 10 (33.3) | - | 3 (10) | 24 (80) | 30 (100) |
| Control (n = 30) | 7 (23.3) | 11 (36.6) | 8 (26.6) | - | 0.00 | 27 (90) | 30 (100) | ||||
| Anaraki and Mirzaei (8) | 2012 | Iran | 15 | Pethidine (n = 39) | 15 (38.4) | 9 (23) | 9 (23) | 15 (38.4) | 4 (10) | 33 (84.6) | 39 (100) |
| Control (n = 39) | 19 (48.7) | 4 (10) | 0.00 | 13 (33.3) | 0.00 | 32 (82) | 39 (100) | ||||
| 25 | Pethidine (n = 39) | 11 (28.2) | 12 (30.7) | 17 (43.5) | 18 (46.1) | 15 (38.4) | 33 (84.6) | 38 (97.4) | |||
| Control (n = 39) | 19 (48.7) | 4 (10) | 0.00 | 13 (33.3) | 0.00 | 34 (87.1) | 39 (100) | ||||
| 30 | Pethidine (n = 39) | 6 (15.3) | 17 (43.5) | 19 (48.7) | 24 (61.5) | 19 (48.7) | 34 (87.1) | 38 (97.4) | |||
| Control (n = 39) | 19 (48.7) | 4 (10) | 0.00 | 13 (33.3) | 0.00 | 34 (87.1) | 39 (100) | ||||
| Khan et al. (33) | 2011 | Iran | 10 | Pethidine (n = 24) | 9 (37.5) | 10 (41.6) | 7 (29.1) | - | 3 (12.5) | 17 (70.8) | 22 (91.6) |
| Control (n = 24) | 18 (75) | 5 (20.8) | 3 (12.5) | - | 0.00 | 18 (75) | 23 (95.8) | ||||
| 25 | Pethidine (n = 24) | 2 (8.3) | 15 (62.5) | 13 (54.1) | - | 3 (12.5) | 18 (75) | 22 (91.6) | |||
| Control (n = 24) | 18 (75) | 5 (20.8) | 3 (12.5) | - | 0.00 | 18 (75) | 23 (95.8) | ||||
| Shrestha et al. (23) | 2007 | Nepal | 10 | Pethidine (n = 30) | 12 (40) | 15 (50) | 11 (36.6) | - | 7 (23.3) | - | - |
| Control (n = 30) | 23 (76.6) | 8 (26.6) | 3 (10) | - | 0.00 | - | - | ||||
| Roy et al. (34) | 2004 | Canada | 15 | Pethidine (n = 20) | 4 (20) | 12 (60) | 9 (45) | - | 10 (50) | - | - |
| Control (n = 20) | 15 (75) | 7 (35) | 2 (10) | - | 0.00 | - | - | ||||
| Mohamed et al. (35) | 2018 | Egypt | 25 | Pethidine (n = 25) | 6 (24) | 16 (64) | 13 (52) | - | 17 (68) | - | - |
| Control (n = 25) | 22 (88) | 8 (32) | 6 (24) | - | 1 (4) | - | - | ||||
Study Characteristicsa
| Authors’ Names | Year | Country | Dose, mg | Pethidine/Control (Number) | Variables | ||
|---|---|---|---|---|---|---|---|
| Time of Sensory Block, min | Time of Complete Motor Block, min | Analgesia Duration, min | |||||
| Farzi et al. (11) | 2014 | Iran | 25 | Pethidine (n = 65) | 4.5 ± 0.51 | 6.24 ± 0.66 | - |
| Control (n = 65) | 5.13 ± 0.29 | 6.86 ± 0.7 | - | ||||
| Atalay et al. (24) | 2010 | Turkey | 35 | Pethidine (n = 20) | 6.5 ± 1.5 | - | 403 ± 43 |
| Control (n = 20) | 6.1 ± 1.4 | - | 185 ± 20 | ||||
| 30 | Pethidine (n = 20) | 6.9 ± 1.7 | - | 315 ± 29 | |||
| Control (n = 20) | 6.1 ± 1.4 | - | 185 ± 20 | ||||
| 25 | Pethidine (n = 20) | 7 ± 1.7 | - | 295 ± 23 | |||
| Control (n = 20) | 6.1 ± 1.4 | - | 185 ± 20 | ||||
| Yu et al. (21) | 2002 | China | 10 | Pethidine (n = 20) | - | - | 234 ± 25 |
| Control (n = 20) | - | - | 125 ± 12 | ||||
| Kouzegaran et al. (25) | 2018 | Iran | 5 | Pethidine (n = 20) | - | - | 139 ± 45 |
| Control (n = 20) | - | - | 87.6 ± 30 | ||||
| Zebetian et al. (28) | 2013 | Iran | 10 | Pethidine (n = 35) | - | 6.95 ± 1.4 | - |
| Control (n = 35) | - | 7.35 ± 1.10 | - | ||||
| Kafle (31) | 1993 | Nepal | 10 | Pethidine (n = 25) | 5 ± 2 | - | - |
| Control (n = 25) | 5 ± 2 | - | - | ||||
| Hong and Lee (32) | 2005 | South Korea | 10 | Pethidine (n = 30) | 4.8 ± 0.2 | 7.1 ± 1.5 | - |
| Control (n = 30) | 5.75 ± 0.3 | 7.2 ± 1.7 | - | ||||
| Shrestha et al. (23) | 2007 | Nepal | 10 | Pethidine (n = 30) | - | - | 510 ± 32 |
| Control (n = 30) | - | - | 96 ± 12 | ||||
| Roy et al. (34) | 2004 | Canada | 15 | Pethidine (n = 20) | 4.5 ± 0.41 | 6.75 ± 0.21 | |
| Control (n = 20) | 5.1 ± 0.39 | 6.95 ± 0.29 | |||||
| Mohamad et al. (35) | 2018 | Egypt | 25 | Pethidine (n = 25) | - | - | 169.2 ± 7.59 |
| Control (n = 25) | - | - | 93 ± 17 | ||||
Study Characteristicsa
| Parameters | Sample Size | Number of Study | I2, % | RR (95% CI) | P |
|---|---|---|---|---|---|
| Shivering (dose ≤ 20) | 720 | 12 | 61.6 | 0.34 (0.23, 0.48) | 0.003 |
| Shivering (dose > 20) | 440 | 8 | 62.9 | 0.18 (0.09, 0.39) | 0.009 |
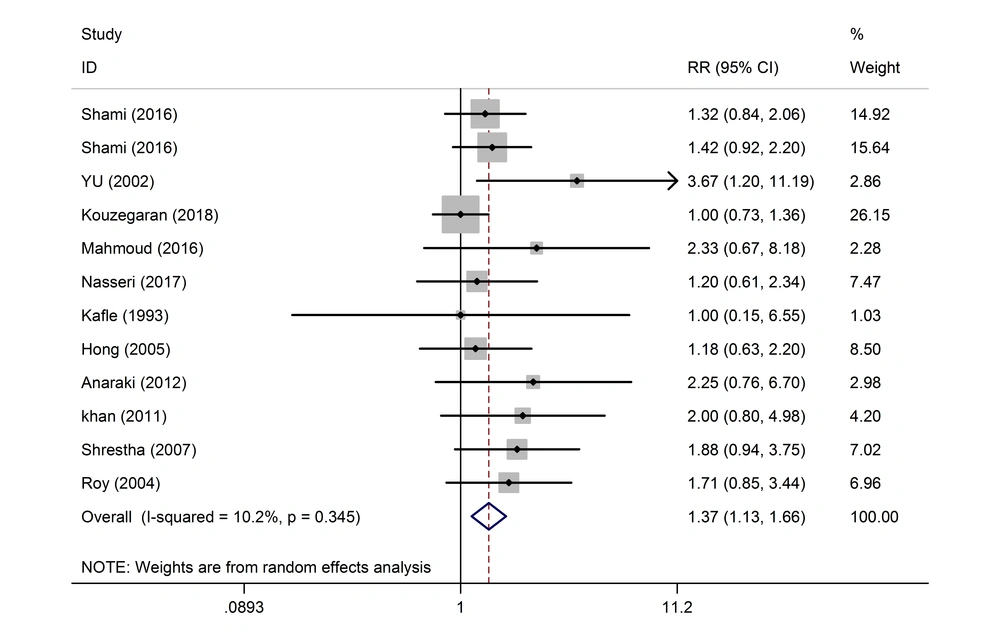
| Nausea (dose ≤ 20) | 690 | 12 | 10.2 | 1.37 (1.13, 1.66) | 0.345 |
| Nausea (dose > 20) | 520 | 9 | 69.8 | 1.33 (0.88, 2.02) | 0.001 |
| Vomiting (dose ≤ 20) | 670 | 12 | 60.5 | 2.02 (1.28, 3.20) | 0.003 |
| Vomiting (dose > 20) | 520 | 9 | 72.8 | 1.57 (0.78, 3.17) | 0.000 |
| Hypotension (dose ≤ 20) | 450 | 7 | 0.00 | 1.00 (0.87, 1.15) | 0.473 |
| Hypotension (dose > 20) | 517 | 8 | 62.8 | 1.01 (0.72, 1.43) | 0.009 |
| Pruritus (dose ≤ 20) | 760 | 12 | 0.00 | 9.26 (4.17, 20.58) | 0.999 |
| Pruritus (dose > 20) | 490 | 8 | 0.00 | 17.77 (7.34, 43.00) | 0.986 |
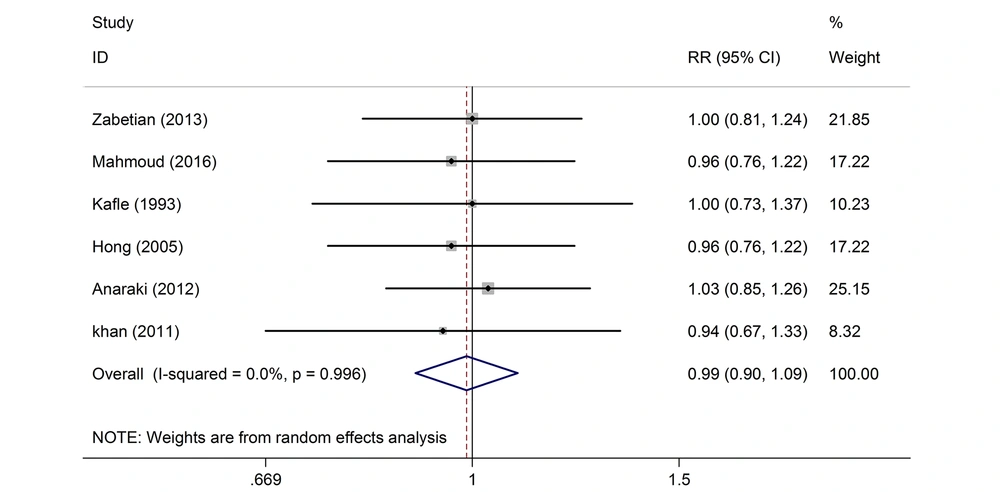
| Apgar > 7 at 1 min (dose ≤ 20) | 370 | 6 | 0.00 | 0.99(0.9, 1.09) | 0.996 |
| Apgar > 7 at 1 min (dose > 20) | 568 | 9 | 0.00 | 0.96 (0.92, 1.02) | 1.000 |
| Apgar > 7 at 5 min (dose ≤ 20) | 340 | 6 | 0.00 | 0.93 (0.87, 1.08) | 0.687 |
| Apgar > 7 at 5 min (dose > 20) | 570 | 6 | 0.00 | 0.99 (0.95, 1.02) | 0.889 |
Estimation of Relative Risk and Confidence Interval 95% in Variables: Shivering, Nausea, Vomiting, Hypotension, Pruritus and Apgar Score
No significant effect was observed in eight of the studies on the effect of the intrathecal pethidine with doses less and more than 20 mg on the maternal hypotension (Table 3). Out of the 12 studies, the effect of the intrathecal pethidine with a dose of ≤ 20 on nausea was not significant in 11 studies except for one study. The final result was significant with a total composition of the 12 studies. The weight of the Shami et al. (10) study was more than other studies. The rate of nausea resulted from the pethidine with dose > 20 was not significant in 9 studies (Table 3 and Figure 3). In the 12 studies, the effect of the intrathecal pethidine with a dose of ≤ 20 on vomiting and postoperative pruritus was significant. Those who had received pethidine at a dose of ≤ 20 experienced pruritus 26% more. In nine of the studies on the effect of intrathecal pethidine with a dose of > 20 on vomiting, not a significant difference was observed. While nine of the studies on the effect of the intrathecal pethidine with a dose of > 20 on pruritus showed a meaningful difference. Here, 77% of those who had received the pethidine at a dose of > 20 showed more pruritus (Table 3).
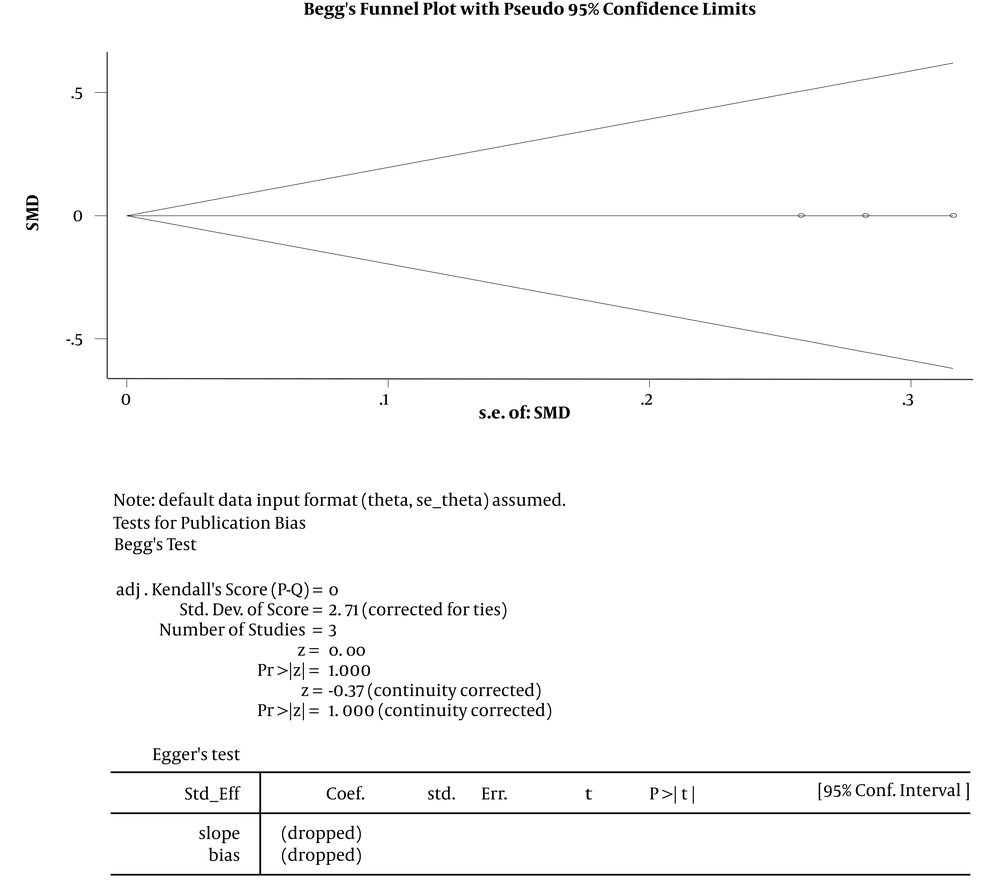
The results of the analysis of the studies with different doses indicated that the intrathecal pethidine had no effect on the neonate Apgar score, maternal sensory and motor block time; and no significant effect was found in the study of these variables (Tables 3 and 4 and Figure 4). In the three studies, the effect of intrathecal pethidine with a dose of ≤ 20 on the duration of the postoperative analgesia was significant. Those who received pethidine had 67% more analgesic duration (Table 4). The funnel plot showed that the effect of bias was not meaningful confirming that the studies with positive and negative results had a chance to print and have been included in the study (Figure 5). The meta-analysis showed that the new studies were less effective (Figure 6).
| Parameters | Sample Size | Number of Study | I2, % | SMD (95% CI) | P |
|---|---|---|---|---|---|
| Time of sensory block (dose ≤ 20) | 150 | 3 | 96.2 | -1.72 (-3.78, 0.34) | 0.00 |
| Time of sensory block (dose > 20) | 210 | 4 | 94.3 | 0.05 (-1.11, 1.22) | 0.00 |
| Time of complete motor block (dose ≤ 20) | 100 | 2 | 96.6 | -4.38 (-9.19, 0.44) | 0.00 |
| Time of complete motor block (dose > 20) | - | - | - | - | - |
| Analgesia duration (dose ≤ 20) | 140 | 3 | 97.9 | 7.67 (1.85, 13.49) | 0.00 |
| Analgesia duration (dose > 20) | - | - | - | - | - |
Comparison of Mean of Variables Time of Sensory Block, Time of Complete Motor Block, and Analgesia Duration in the Pethidine and Control Groups
4. Discussion
In this study, we evaluated the effects of different doses of the intrathecal pethidine (5 to 40 mg) on the maternal and newborn parameters during the cesarean section under the spinal anesthesia. Our study showed that administration of the intrathecal pethidine in the cesarean patients with under the spinal anesthesia can reduce the postoperative shivering so that the more the doses of the pethidine, the more the reduction in the shivering. The intrathecal pethidine in the pethidine group increases the relative risk of nausea, vomiting, and pruritus after the surgery in the patients with the cesarean delivery. With the increased doses of pethidine, the relative risk of pruritus gets higher in the cesarean section. The results of the analysis of the studies with different doses indicated that the intrathecal pethidine had no effect on the newborn Apgar score, mother’s hypotension, and the maternal time of the motor and sensory block. On the other hand, mothers of the cesarean section who had received pethidine under the spinal anesthesia had more duration of analgesia after the surgery.
In a meta-analysis by Lin et al. (20) on the effect of the intrathecal pethidine (5/25 mg) on various surgeries, they revealed that a minimum dose of pethidine reduced significantly shivering and increased the demand for sedatives; however, the risk of nausea and vomiting is increased in the patient. Studies carried out by Popping et al. (36), Shami et al. (10), Rastegarian et al. (9), and Nasseri et al. (30) all suggested the intrathecal anti-shivering effect of the opiates, that are consistent with the results of the present study.
Shivering during the spinal anesthesia owns a multi-factor mechanism. The sympathetic block, due to the spinal anesthesia, causes disturbance compensatory vasoconstriction and automatic adjustment below the level of blockage and slows down the thermoregulation, which leads ultimately to the vasodilatation and hypothermia. All these factors contribute finally to shivering (37, 38).
Pethidine is one of the most commonly used drugs for treating and preventing both shivering and pain, which is more effective in controlling these two rather than the other sedatives (20, 39). Although the mechanism of the effect of pethidine on shivering and pain control is not clear, it may be probably due to its direct effect on the temperature regulation center or its agonistic effects on the sedative receptors of µ and Қ (40, 41).
In the study of Shami et al. (10), Farzi et al. (11), and Rastegarian et al. (9), there was no statistically significant difference in nausea and vomiting between the two pethidine and control groups. On the contrary, Lin et al. (20) reported the increased risk of nausea and vomiting in their meta-analysis, which is consistent with the results in the present study.
In our study out of 12 studies on the effect of intrathecal pethidine with a dose of 20 mg or less on nausea, only one study was significant while the rest 11 ones were not. On the whole, as far as the 12 studies are concerned, the difference was meaningful. One of the benefits of the meta-analysis studies is that in the separate studies due to the low sample size in each study, even high effect sizes may not be significant. For example, nausea had an effect size greater than 2 in 4 studies which was not considered as significant due to the low number of the samples in each study, but when the 12 studies were combined altogether, because of the increases sample sizes, the effect size turns out significant showing that nausea was 37% higher in the patients taking pethidine. The same applies to vomiting.
Like other narcotics, pethidine has side effects such as respiratory depression, hypotension, nausea, vomiting, decreased gastrointestinal motility, and physical dependency (41). The increase in nausea and vomiting just followed by the administration of intrathecal pethidine is resulted from multiple issues. Firstly, the intrathecal pethidine may act as an independent analgesic. Therefore, when it is combined with a high level of anesthesia, it can lead to a decrease in systemic blood pressure along with vomiting and nausea. On the other hand, pethidine like other narcotics has central effects on nausea and vomiting (20, 21).
In a series of studies (10, 24), the rate of pruritus following the intrathecal pethidine was significant, while it was not significant in some other studies (9, 11, 32). This difference may be due to the administration of the pethidine dose. In our meta-analysis, with an increased dose of pethidine, the relative risk of pruritus was increased.
In the study of Zebetian et al. (28), Hong and Lee (32), and Anaraki and Mirzaei (8), there was no significant difference in the newborn Apgar score, which is quite consistent with the present study. There was no significant difference in the duration of the sensory and motor blocks in the mothers with the cesarean section, which is in line with the studies performed by Hong and Lee (32) and Roy et al. (34). In our study, the mothers who had received the intrathecal pethidine had longer duration analgesia after the surgery, which was consistent with the results reported by Kouzegaran et al. (25), Shrestha et al. (23), and Yu et al. (21).
4.1. Limitations and Strengths
The small number of studies in this meta-analysis is one of the limitations; however, there has been no bias in the inclusion of the studies in this meta-analysis. The absence of language constraints in entering the studies and including the entire parameters of the studies in the meta-analysis are among the strengths of this study.