1. Background
Advanced medical care and increased geriatric population in recent years have led to an increased number of glaucoma cases. Subsequently, the number of glaucoma surgeries has increased to solve this problem (1, 2).
Local anesthesia, such as the peribulbar block, is used to decrease the interval from admission to discharge and reduce burdens on both medical teams and patients. However, these blocks may increase intraocular pressure (IOP), thus limiting their use in eye surgery. Intraocular pressure can be decreased by dexmedetomidine at both histological and clinical levels (3, 4). Likewise, it prolongs the block duration and has a sedative effect if used as an additive to local anesthesia, as shown in many studies (5, 6). These effects have been tested by adding dexmedetomidine to the block via many routes such as intravenous and intramuscular routes or even concurrent use with local anesthesia in the same syringe (5, 7, 8). Until now, there is no consensus on the dose and route of dexmedetomidine administration to achieve the above-mentioned effects.
The effect of dexmedetomidine on the IOP decrease has not yet been examined in eyes with glaucoma. Hypothetically, dexmedetomidine, if used locally as an additive to local anesthesia, can decrease IOP in the glaucomatous eye to the extent that will lead to proper surgical conditions and better surgical outcomes.
2. Methods
This is a prospective triple-blinded randomized-controlled trial with three parallel groups registered in the clinical trial registry (NCT02846090). After the ethics committee approval, we enrolled all patients scheduled for glaucoma surgery (subscleral trabeculectomy) in both Cairo University Hospital and Research Institute of Ophthalmology Hospital. Each patient signed a consent form after being briefed on study procedures and safety precautions.
The inclusion criteria included patients with ASA I-III, age of 25 to 80 years, established high IOP, and signposted for candidate for surgical intervention. On the other hand, the exclusion criteria included unwilling patients, patients with known allergy to any study drugs, ASA IV or more, age below 25 or above 80, mental disorders such as dementia, communication barriers such as deafness and/or psychological diseases, inability to be placed horizontally, INR above 1.7, or major coagulation defect.
The patients were randomly assigned to three groups. In Group D50, 50 µg of dexmedetomidine in a volume of 0.5 ml was added to 9.5 mL of a mixture of local anesthesia composed of 5 mL of 0.5% bupivacaine, 4.5 mL of 2% lidocaine, and 150 IU hyaluronidase. In group D25, 25 µg of dexmedetomidine in a volume of 0.5 mL was added to the above-mentioned mixture. In group C (control group), 0.5 mL of saline was added instead of dexmedetomidine to the above mixture.
All allocated patients had an intravenous line, connected to basic monitors (non-invasive blood pressure, oxygen saturation, and ECG) and the nasal cannula with an oxygen flow of four liters. With the operative eye marked and in the supine position, the peribulbar block was given using 26-gauge, 13-mm needles through the pre-cleaned aseptic skin (by alcohol) in the inferotemporal quadrant. The needle advanced parallel to the orbital floor, and 7 mL of the local anesthetic mixture was given after a negative aspiration test. Then, 3 mL extra mixture was given through the medial epicanthus, followed by compression and message to the ocular ball using sterile gauze for six minutes. The patients in all groups were monitored for the following items.
1. Intraocular pressure (primary outcome) before local anesthesia, after rubbing and before draping, and after operation using a Goldmann applanation tonometer.
2. Beginning of the block: The interval between needle withdrawal and complete or partial akinesia. Patients with full motor movement or no anesthesia indicated the failure of the block and were excluded from the study and follow-up.
3. Sedation level every 10 minutes and for two hours using the Ramsay Sedation Scale (RSS). The RSS is used for the assessment of patient sedation as follows: anxious agitated and restless = 1 point; cooperative, oriented, and tranquil = 2 points; responding only to verbal commands = 3 points; brisk response to light glabella tap or loud auditory stimulus = 4 points; sluggish response to light glabella tap or loud auditory response = 5 points; and no response to light glabella tap or loud auditory response = 6 points.
4. Duration of the block: It was regarded as the ability of the patient to move both eyes simultaneously with no corneal reflex.
5. Patients’ hemodynamics (blood pressure, oxygen saturation, and heart rate) throughout the procedures and two hours later looking for any adverse effect.
No sedation was given throughout the procedures, and the providers fully explained the procedures and reassured the patients. In the case of failure of the block, the patient would be shifted to general anesthesia and excluded from the study.
The sample size was calculated based on a method described by Lerman (9) and data from previous studies (10, 11). The adequate sample size was 29 people for each group by assuming a 30% variance between each group as clinically significant and considering the alpha value of 0.05 and a power of 90%. With an expected dropout rate of 20%, we decided to increase the number of patients in each group to 35.
Randomization was done using a randomized computer-generated sequence. This was done by an investigator not involved in the procedure. The investigator who prepared the local anesthetics mixture also was not involved in the surgery or follow-up measurements. The investigator, who did the follow-up procedures, was not involved in the surgery. Therefore, the study was triple-blinded to patients, investigators, and persons involved in follow-up.
2.1. Statistical Methods
The data were described by mean and standard deviation (SD). According to the type and distribution of the data, we used the median and ranges or frequencies (number of cases) and percentages. The ANOVA test was used to compare the numerical data between the groups. The categorical data were analyzed using the chi-square (χ2) test. If the frequency was expected to be less than 5, the Exact test was used. The P values of less than 0.05 were considered significant. All statistical analyses were done using the Statistical Package for the Social Sciences (SPSS) software (IBM Corp., Armonk, NY, USA).
3. Results
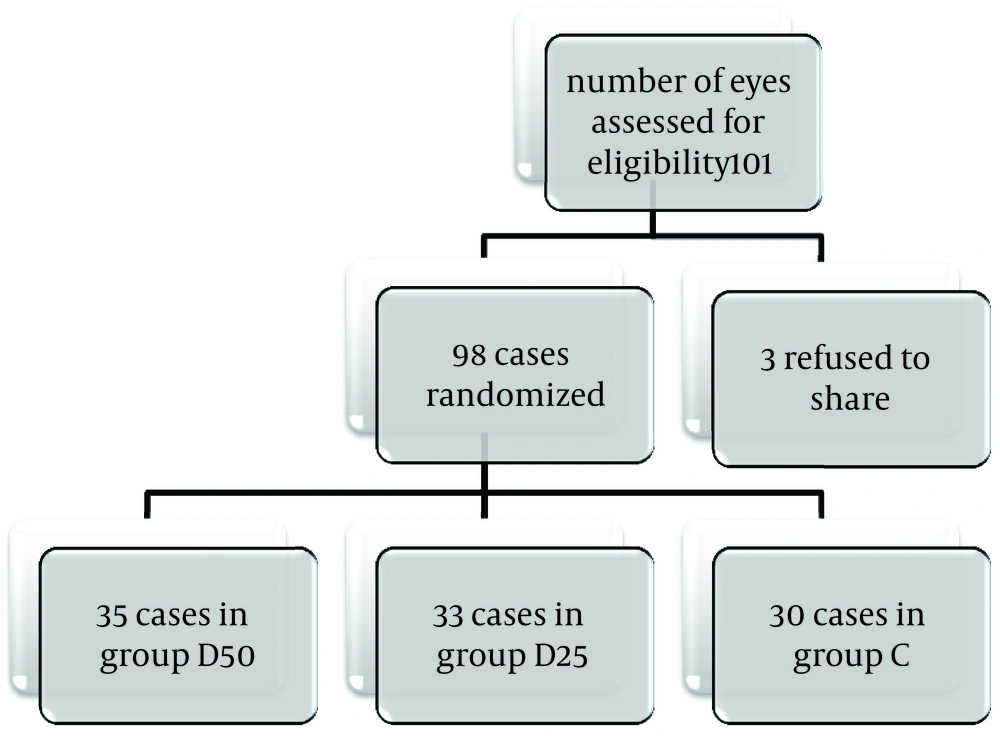
This study enrolled 101 patients’ eyes that were indicated for glaucoma surgery. Three patients refused to participate in the study. Thus, 98 willing patients were randomly assigned to either group of D50, D25, or control (C). The study was running over six months from 1, August 2016 to 27, February 2017. No cases (or eyes) were dropped out because of the failure of the block or something else (Figure 1).
There were no statistical differences between the three groups in the demographic data (Table 1). Also, there were no statistical differences between the three groups regarding the type and duration of surgery. Regarding OIP at pre-injection, after injection, and the end of the surgery, there were no statistical differences (P > 0.05) between the groups during the whole procedure and even after the end of the surgery (Table 2). Dexmedetomidine did not decrease IOP in the glaucomatous eyes.
| Characteristic | D50 (N = 35) | D25 (N = 33) | C (N = 30) | P Value |
|---|---|---|---|---|
| Age (y) | 56.14 ± 7.923 | 54.25 ± 5.23 | 55 ± 6.47 | 0.537 |
| Sex (M/F) | 25/10 | 23/10 | 18/12 | 0.332 |
| Patients height (cm) | 165 ± 7.9 | 167 ± 8.3 | 170 ± 6.3 | 0.368 |
| Patients Weight (kg) | 89 ± 5.62 | 79 ± 6.358 | 85 ± 4.96 | 0.689 |
| Patient status ASA (II/I) | 30/5 | 29/4 | 27/3 | 0.716 |
| Duration of surgery (min) | 32.25 ± 15 | 29.25 ± 9.85 | 31.85 ± 11.32 | 0.25 |
| Type of surgery (SST/valve) | 25/10 | 22/11 | 21/9 | 0.900 |
Demographic and Clinical Characteristicsa
| Intraocular pressure (mmHg) | D50 (N = 35) | D25 (N = 33) | C (N = 30) | P Value |
|---|---|---|---|---|
| Pre-injection | 27.71 ± 2.527 | 27.25 ± 3.53 | 26.2 ± 3.576 | 0.058 |
| After injection | 29.71 ± 1.69 | 30.25 ± 2.36 | 29.4 ± 3.756 | 0.657 |
| At the end of the surgery | 10.86 ± 1.478 | 10.75 ± 1.63 | 10.6 ± 1.589 | 0.502 |
Intraocular Pressure through the Procedurea
Regarding the dynamics of the peribulbar block (onset and duration), dexmedetomidine had a great impact; it decreased the onset and prolonged duration in both study groups. The onset of the block decreased from six minutes in the control group to four minutes in the D25 group (P = 0.00) and it continued to decrease to 3.57 minutes in the D50 group (P = 0.00) with a dose-response curve. Moreover, the duration of the block increased significantly in a dose-response manner; it was 160 ± 20.36 minutes in the control group that increased to 223.36 ± 25.63 minutes in the D25 group and 252.36 ± 36.82 minutes in the D50 group with P = 0.00 (Table 3).
Regarding the sedative effect of dexmedetomidine, there was no sedative effect in the three groups and all patients were fully conscious and scored 2 on the RSS.
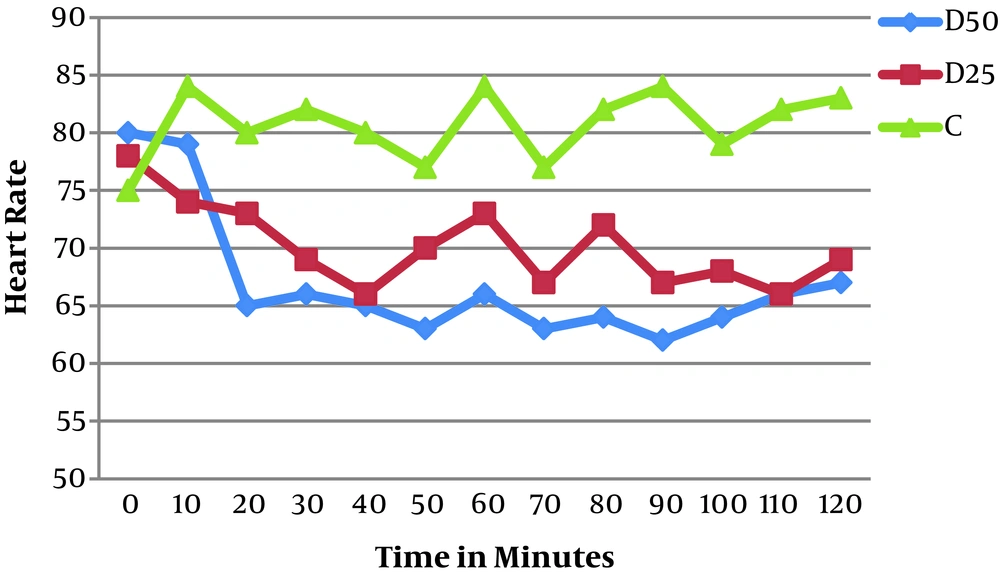
Regarding the hemodynamics (blood pressure and heart rate), there was a statistically significant difference between the groups in the heart rate and it was lower in both study groups than in the control group (P = 0.031); however, this difference was not clinically significant, as it was 65 beats per minute in group D50, 70 beats per minute in group D25, and 79 beats per minute in group C. However, there were no statistical differences between the three groups in blood pressure (P = 0.512) (Table 4; Figures 2 and 3).
4. Discussion
This study showed that dexmedetomidine, as an additive to bupivacaine in the peribulbar block, did not affect intraocular pressure. Moreover, it had no sedative effect on patients. On the other hand, it prolongs the duration of the block and shortens the time to the onset. It decreases the heart rate but without a clinical significance. This may be explained by the patients’ co-morbidities, such as hypertension that was treated with heart rate-controlling medications. Dexmedetomidine has been tested in many studies alone or as an adjuvant to bupivacaine in the peribulbar block (3, 7, 10, 11). This combination aims to prolong the block duration to hasten the onset, decrease intraocular pressure, and look for its sedative effects.
Intraocular pressure was not the main target in these studies. However, the authors found that it markedly decreased intraocular pressure. It is unknown if dexmedetomidine can be used as an adjuvant to bupivacaine aiming at getting its benefits as a sedative and augmenter and to decrease IOP in the glaucomatous eye. Unfortunately, the drug failed to decrease IOP in the diseased eye. Likewise, it failed to sedate the patients.
This failure may be explained by the present pathology that could be overcome by this simple nonspecific medicine. This finding is supported by a study by Lili et al. (8) that tested dexmedetomidine in vitreoretinal surgery through the intravenous route. Although the authors used a different route, dexmedetomidine showed no effect on IOP. Moreover, it did not affect the patients’ sedation level; this may be because of the small dose and the administration route. Although it succeeded in other studies to achieve a level of sedation, this fact is not established widely, possibly because of the different routes and small doses (5, 6, 12, 13). However, Channabasappa et al. and Abdelhamid et al. tested dexmedetomidine in cataract surgery as an adjuvant to bupivacaine in the peribulbar block and noticed a sedative effect through this route. They used the same doses and the same route; this contradiction may be explained by the type of pathology that may have an impact on drug absorption (10, 11).
Dexmedetomidine is a very interesting drug with many indications and effects in the form of analgesia and sedation. Moreover, it has proven to be an effective additive in many studies and through many routes (14-16). These characteristics are attributed to its mechanism of action as it works as an alpha-1 receptor agonist, which has sedative and analgesic effects with the advantage of no respiratory depression. This effect is due to decreasing noradrenaline and increasing Gamma-aminobutyric acid in the brain stem and ventrolateral preoptic nucleus, respectively.
4.1. Conclusions
This study concludes that dexmedetomidine is not helpful in glaucomatous eyes neither for controlling IOP nor for sedation. The limitation of this study is that it compared dexmedetomidine through only one route and only at two small doses. Further studies are needed to test its effect at higher doses through the same route or other routes.