1. Background
Pain is one of the most common clinical problems, especially in patients after surgery. Pain reduction can increase patient satisfaction and decrease postoperative hospitalization (1, 2). Pain receptors in the damaged tissues can transfer signals that cause a cascade increasing the central and peripheral nervous response. Sensory processing changes cause a severe response to stimuli and pain. Blocking these signals may inhibit cascade progression and reduce postoperative pain (3). In the preemptive analgesic method, painkillers are used before painful stimulation, whereas in the conventional analgesic method, they are used after pain development. This method was first introduced in animal studies to reduce postoperative pain by Wall in 1988 (4) and Woolf in 1991 (5). Postoperative pain can increase the hospitalization time and the risk of infection. Acute postoperative pain is observed in 10% - 65% of patients after surgery (6, 7). A common approach for pain management after surgery is to relieve the pain that has already occurred. On the other hand, there is a growing popularity for preoperative prescriptions of painkillers to reduce postoperative pain (8). Tissue damage during the surgical procedure causes the release of inflammatory chemical mediators. Some of these mediators, including histamine, acetylcholine, and bradykinin, cause pain, and some others, such as prostaglandins, cause hyperalgesia, which is characterized by a decrease in pain threshold and an increase in sensitivity to extrinsic stimuli (9). It is hypothesized that preventive analgesia leads to inhibition of central sensitization caused by an inflammatory stimulus of surgery (10). Therefore, this may spare patients from long-term side effects and chronic pain, which usually lasts months after surgery (11). Chronic pain can lead to physical, mental, and social disabilities (11). Therefore, postoperative pain management is very important. Reducing postoperative pain increases satisfaction in most patients and decreases hospitalization as well as immobility complications. Thus effective preventive analgesia improves quality of life and reduces financial costs (12).
2. Objectives
The aim of the current clinical trial is to use a specific method of a two-stage preemptive analgesic method after 6 major surgeries by administration of pregabalin, acetaminophen, naproxen, and dextromethorphan (PAND) to develop a new method to reduce postoperative pain and reduce drug use in patients.
3. Methods
3.1. Patients
A double-blind study was conducted on 61 subjects that were aged between 18 and 80 years, and had an American Society of Anesthesiology (ASA) physical status between 1 and 3, who were referred to the operating room of Imam Khomeini Hospital affiliated to Tehran University of Medical sciences for one of the elective surgeries including radical prostatectomy, radical nephrectomy, radical cystectomy, Whipple procedure, total colectomy, and retroperitoneal lymph node dissection (RPLND) (Figure 1). This study was approved by the ethics in the research committee of Tehran University of Medical Sciences (code: IR.TUMS.IKHC.REC.1398.017). Signed informed consent was obtained from all participants of the study. Moreover, the current trial was registered in the Iranian registry of clinical trials (IRCT) at 2019-04-19, with IRCT code: IRCT20181219042046N1.
Exclusion criteria included opioids addiction, history of antidepressant and anticonvulsant drug use in the last 6 months, nonelective surgery, ASA physical status more than 3, history of drug sensitivity to preemptive analgesia agents used in this study (PAND), and the patients who did not extubate after surgery.
All patients received a combination of PAND with a small amount of water one hour before entering the operating room. After transferring to the ward, the patients were randomly divided into two groups for receiving the second dose. Simple randomization, based on a random number table, was used to randomize the participant. One (included odd numbers) that was given the second dose of PAND combination and one (included even numbers) that did not receive any medication. Only the research guide was aware of the prescribed drug and none of the prescribing persons and patients were aware of the prescribed medicine. In the first group, a combination of pregabalin (151 mg), acetaminophen (one gram), naproxen (511 mg), and dextromethorphan (31 mg) was administered one hour before surgery and a second dose of the same combination was given within 6 hours after discharge from recovery. In the second group, the same combination is prescribed only one hour before surgery. All patients were transferred to the operating room and received 2 mg midazolam in addition to 2 mg/kg fentanyl as premedication. Then anesthesia was induced with 2 - 3 mg/kg thiopental sodium in addition to 0.5 mg/kg atracurium.
Anesthesia was followed with 0.8 - 1.2 isoflurane and a 50:50 mixture of nitrous oxide and oxygen. Before surgical incision, 1 µg/kg fentanyl was injected, and atracurium, as well as fentanyl, were administered as needed for maintenance during anesthesia.
After skin closure, 40 µg/kg neostigmine and 20 µg/kg atropine were given to restore the neuromuscular block. The duration of each surgery was recorded in minutes from the moment of the incision until wound closure. Following extubation, the patients were transferred to recovery. After achieving complete consciousness, the pain intensity was assessed by the universal pain assessment tool (UPAT) (time zero). In addition, Pain intensity was assessed at 2, 4, 6, 8, 12, 24, and 48 hours postoperatively. The type of pain (somatic pain vs. acute neuropathic pain) was also recorded. Morphine was prescribed according to the protocol for pain scores greater than 4 (UPAT > 4) to control the patient’s pain. Patients’ first need for analgesic medication, cumulative doses of morphine used to control pain, and drug induced complications were recorded in the postoperative period.
Diagnosis of somatic pain and acute neuropathic pain was performed based on the standard definition for somatic and neuropathic pain (13) and the nature of the pain that the patient felt and described as well as our findings on physical examination.
3.2. The Randomization and Blinding Method
The randomization method was as follows: Patients were divided into two groups randomly according to the random number table to receive the second dose after being transferred to the ward. Accordingly, a random number was assigned to each patient (individual or paired). The combination of the drug and the placebo were packed in similar white bags by the research guide and were used as the second dose.
The group with odd numbers received a second dose of the drug compound, and the other group (even numbers) received the placebo. The only person who knew the contents of the envelope was the research guide, and the patients, the administrators, and outcome evaluators were blinded.
3.3. Statistical Analysis
Statistical analysis was performed with the Statistical Package for Social Sciences (SPSS) version 25.0 statistics software.
Central and dispersion indices were calculated for quantitative data (mean, standard deviation, and amplitude). An independent t-test was used to compare the effect of treatment in the two groups, Descriptive statistics and regression analysis was used for data analysis (ASA-ps, gender, type of surgery, age over 75 years, units of blood consumption, and duration of anesthesia).
4. Results
Demographic data in the two groups (The one dose and two dose groups) are shown in Table 1. There was no statistically significant difference in the demographic data between the two groups.
| Variable | Group I (N = 30) | Group II (N = 30) | P Value |
|---|---|---|---|
| Age, y | 55.50 ± 13.73 | 58.20 ± 12.67 | 0.431 |
| BMI, kg/m2 | 25.16 ± 4.02 | 25.41 ± 3.72 | 0.802 |
| Sex, M/F | 25/5 | 22/8 | 0.347 |
| ASA | 0.186 | ||
| I | 17 (56.7) | 10 (33.3) | |
| II | 9 (30.0) | 13 (43.4) | |
| III | 4 (13.3) | 7 (23.3) | |
| Type surgery | 0.054 | ||
| Prostatectomy | 6 (20) | 10 (33.3) | |
| Nephrectomy | 1 (3.3) | 5 (16.7) | |
| Cystectomy | 9 (30.0) | 3 (10.0) | |
| Whipple | 5 (16.7) | 2 (6.6) | |
| Colectomy | 8 (26.7) | 5 (16.7) | |
| RPLND | 1 (3.3) | 5 (16.7) |
Abbreviations: ASA, American Society of Anesthesiology; BMI, body mass index; SD, standard deviation.
aValues are expressed as mean ± SD or No. (%).
bStatistically significant difference: P < 0.05.
Table 2 shows the mean pain scores in both groups at times 0, 1, 2, 6, 12, 24, and 48 hours postoperatively. Mean pain scores were significantly different in two groups at 2, 12, 24, and 48 hours after surgery (P < 0.05).
| Timem h | Group I | Group II | P Value |
|---|---|---|---|
| 0 | 0.43 ± 1.35 | 0.50 ± 1.52 | 0.784 |
| 1 | 1.27 ± 2.05 | 1.0 ± 1.70 | 0.688 |
| 2 | 3.50 ± 2.67 | 2.13 ± 2.31 | 0.043 |
| 6 | 2.57 ± 2.67 | 1.90 ± 2.17 | 0.304 |
| 12 | 4.57 ± 2.68 | 1.37 ± 1.93 | < 0.001 |
| 24 | 3.27 ± 2.149 | 2.07 ± 2.11 | 0.027 |
| 48 | 2.10 ± 1.78 | 1.03 ± 1.60 | 0.016 |
aValues are expressed as mean ± SD.
The mean pain score changes from 0 to 1, 1 to 2, 2 to 6, 6 to 12, 12 to 24, and 24 to 48 hours after surgery are shown in Table 3. Based on the results, there was a significant difference between the two groups at 6 to 12 and 12 to 24 hours after surgery (P < 0.05). There was no significant difference in mean pain scores between the two groups at other times (P > 0.05).
| Postoperative Time, h | Group I | Group II | P Value |
|---|---|---|---|
| 0 - 1 | 0.83 ± 1.46 | 0.50 ± 1.10 | 0.324 |
| 1 - 2 | 2.23 ± 3.26 | 1.13 ± 2.99 | 0.179 |
| 2 - 6 | -0.93 ± 4.00 | -0.23 ± 3.25 | 0.461 |
| 6 - 12 | 2.00 ± 3.62 | -0.53 ± 3.18 | 0.006 |
| 12 - 24 | -1.30 ± 2.94 | 0.70 ± 3.07 | 0.013 |
| 24 - 48 | -0.30 ± .87 | -0.53 ± 1.00 | 0.343 |
aValues are expressed as mean ± SD.
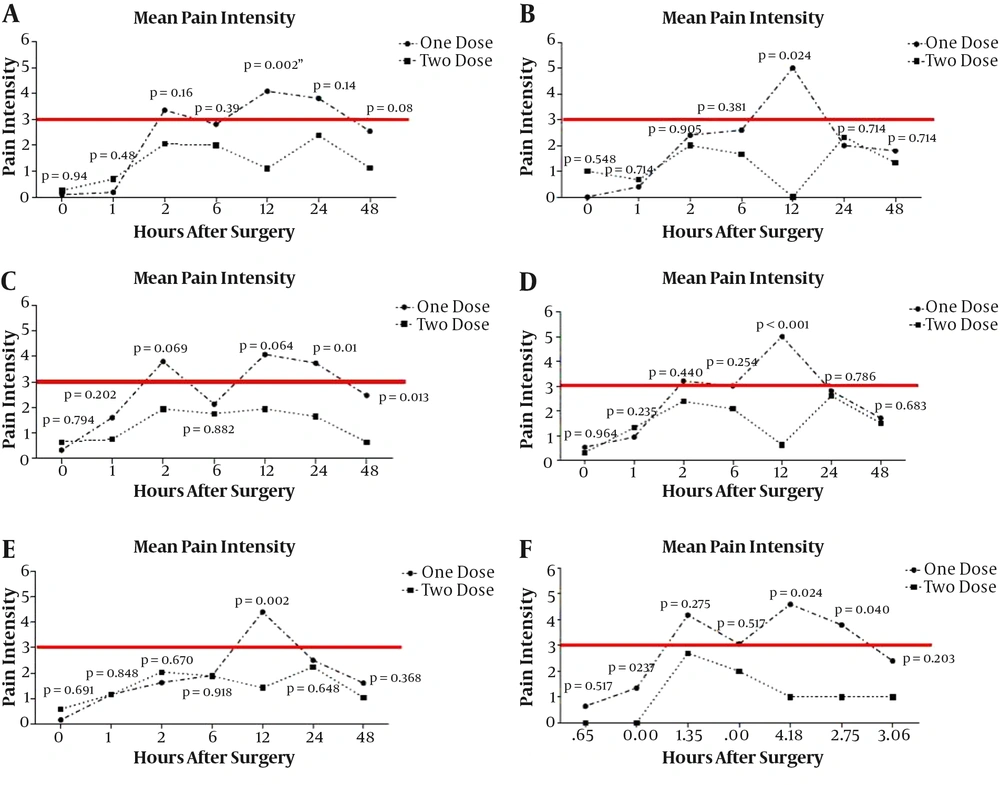
Figure 2 shows the mean pain scores in both groups at times 0, 1, 2, 6, 12, 24, and 48 hours postoperatively. Mean pain scores were significantly different in two groups at 2, 12, 24, and 48 hours after surgery (P < 0.05).
Comparison of pain intensity at 0, 1, 2, 6, 12, 24, and 48 hours after surgery in patients over 65 years, over 90 kg, with BMI less than and greater than 25, with anesthesia duration less than 4 hours and longer than 4 hours among two groups are summarized in Figure 3.
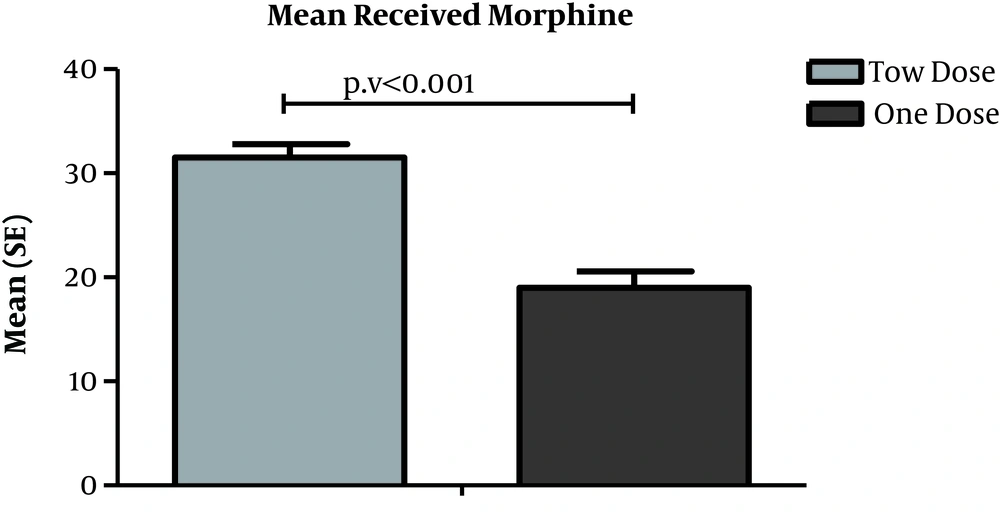
4.1. Determination and Comparison of Total Opioid Consumption
According to the results shown in fig 1, the mean opioid consumption was 31.50 ± 7.08 and 19.0 8 ± 8.55 in the first and second groups, respectively. There was a statistically significant difference between the two groups in terms of opioid consumption (P < 0.001). The total opioid consumption in the second group (with the second administration of PAND) was lower than the first group.
5. Discussion
In the current study, the effect of single-dose versus two-dose administration of PAND combination was evaluated among patients who had undergone one of the surgeries (including nephrectomy, cystectomy, prostatectomy, colectomy, Whipple, and RPLND). Our findings showed the preemptive analgesia with a second dose of PAND as an effective method for reducing pain after surgery. Preemptive analgesia has certain clinical benefits, including reducing psychological distress, sleep disturbances, and the need for analgesics with the associated complications (14-17), as well as faster recovery and earlier and safer discharge (18).
Gabapentinoids, including pregabalin, have traditionally been used as anti-epileptic drugs and are currently widely used to relieve neuropathic pain. It decreases the number of pain signals that are sent out by injured nerves, indicating its analgesic effect (19). Acetaminophen has an anti-inflammatory effect via inhibition of cyclooxygenase enzymes 1 and 2 (COX1 and 2) and is extensively prescribed for pain management. Naproxen is also inhibiting COX) 1 and 2 enzymes and is a nonsteroidal anti-inflammatory drug (NSAID) (20, 21). Moreover, Dextromethorphan inhibits the action of, the N-Methyl-D-spartate receptor (NMDAR) and also plays a role in neuropathic pain reduction. It may have an analgesic effect on postoperative acute pain (22, 23). The preoperative analgesic approach may minimize postoperative pain by a variety of complex mechanisms. The COX2 inhibitors inhibit cyclooxygenase and reduce prostaglandin production (24), and provide analgesia by reducing the sensitivity of peripheral pain receptors to mechanical stimuli (25).
In this study, postoperative pain was significantly reduced for the group that received the second dose of PAND postoperatively. The mean pain scores were significantly different in two groups at 2, 12, 24, and 48 hours after surgery (P < 0.05) and were significantly lower in the second group than the first group.
In the current medical approach, opiates are commonly used to control postoperative pain. These can cause many side effects, including nausea, vomiting, and itching. In addition, using opiates has shown insufficient efficacy in postoperative pain management (26). However, in the present study, no side effects were observed. There is increasing evidence suggesting the benefits of a variety of pharmacological agents that are used before surgery to reduce postoperative pain. These include opiate analgesics (27-30), nonsteroidal anti-inflammatory drugs (31-33), neuromodulatory agents (34-37), and clonidine (24, 25, 38). In this trial, the total dose of administered opioid was also lower for the two-dose group compared with the one-dose group (P < 0.001). This is consistent with a prior study on the effects of preemptive analgesia (39), which has suggested that using a combination of PAND as preventive analgesia can decrease the need for opioid consumption and improve pain control.
In a previous study, preemptive acetaminophen was associated with increased analgesia and decreased postoperative analgesic consumption (40). In addition, it has been shown that multi-modal analgesics (gabapentin with NSAIDs or a combination of acetaminophen with NSAIDs) are superior to either of these two alone (41, 42) in terms of decreasing pain after surgery.
In a study conducted to evaluate the effect of oral pregabalin on the duration and quality of postoperative analgesia in spinal anesthesia, it was shown that oral pregabalin extended the duration of postoperative analgesia and reduced morphine intake. Moreover, it improved sleep quality during the first postoperative night (43). In another study aimed at investigating the effect of prophylactic use of oral pregabalin-acetaminophen-naproxen on pain control and morphine usage in patients who had undertaken laparotomy, patients in the PAN group (pregabalin 150 mg, acetaminophen 1 g, and naproxen 250 mg) experienced lower pain and the morphine use was also significantly lower than the control group (44). A previous study on the effect of prophylactic analgesia in women undergoing abdominal hysterectomy suggested the higher satisfaction scores of patients who received a combination of gabapentin and paracetamol compared to those who received gabapentin alone. The authors also claimed decreased narcotic requirements among patients who received preemptive narcotic and nonnarcotic medications prior to hysterectomy (45). The inability to assess the incidence of chronic pain due to time constraints was one of the limitations of the current study. In summary, several agents are examined for preventive analgesia (44). Significant opioid sparing and a decreased pain score following preemptive gabapentin was also reported in several other studies (46-48).
5.1. Conclusions
To conclude, in the current study, the PAND combination was used to pain management. Our results show that preemptive analgesia with a second dose of PAND is an effective method for reducing pain and morphine consumption after surgery. The prerequisite for making preemptive analgesia guidelines is more research in various surgeries.