1. Context
Opioid use disorder (OUD), a major source of morbidity and mortality globally, is frequently linked to opioids given around the time of surgery (1-3). Perioperative period, however, is markedly heterogeneous and the diverse providers, involved at specific phases of perioperative care, use opioids distinctly. Moreover, the drivers of opioid misuse are diverse, and are at-play dissimilarly, throughout the perioperative period (4). Centered on these distinctions the perioperative period can be categorized into four specific phases: (1) the intra-operative stage; (2) the immediate post-operative or recovery period; (3) the in-hospital or in-house phase after the immediate postoperative recovery; and (4) the out-patient stage. Intraoperatively, and in the immediate postoperative recovery periods, the patients are managed mainly by anesthesia providers, while opioids for out-patient analgesia after surgery are prescribed, generally, by a wide range of non-anesthesia providers. Our a-priori hypothesis, based on the existing literature (4, 5), and the reasons presented in this article, is that the risk of OUD is minimal from opioids given in the intraoperative and recovery periods, than when prescribed for out-patient analgesia after surgery; scalable from least for the intraoperative to most for the out-patient phase. This dissimilar potential for OUD may ensue dissimilar recommendations for the distinct providers involved.
2. Objectives
The primary purpose of this review, therefore, was to evaluate the literature for the risk of OUD from opioids given at these specific perioperative stages. To our knowledge no existing review on the topic has scrutinized the literature similarly.
3. Methodology
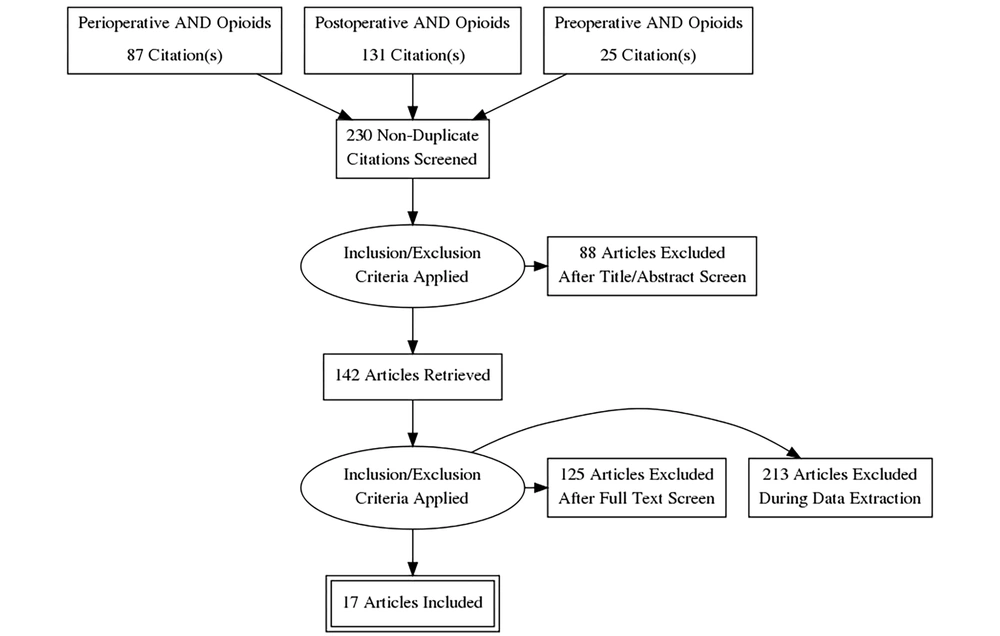
Search Strategy: Following the preferred reporting items for systematic reviews and meta-analysis (PRISMA) guidelines, we performed PubMed, Ovid Medline, and Embase searches using the terms “opioids”, and “addiction” combined with “perioperative”, “post-operative” and “preoperative”, while using no limits. Bibliography of the pertinent articles was also hand searched and the relevant articles were reviewed (Figure 1).
Study Eligibility Criteria: Only the clinical studies in English language, which linked perioperative opioid use to OUD were included in the review. Basic science and animal model studies, review articles, case reports, consensus guidelines, expert opinions, editorials, and any duplicate articles were excluded.
Data Extraction: The abstract of each study retrieved was reviewed and studies meeting the eligibility criteria were selected for the review. Instances where the information attained was ambiguous full text was reviewed and any disagreements were resolved by consensus.
Data Extracted: To determine the quality of the literature reviewed and strength of the evidence presented the type of clinical study i.e. a randomized controlled trial, prospective study, retrospective data review or a case series was ascertained. Since all the studies retrieved were observational in nature their further grading in terms of adequacy of randomization, blinding, nature of control groups and dropout rate was not possible. For reasons presented in the introduction the specific perioperative stage of opioid use i.e. intraoperative, immediate postoperative or recovery, in-patient, or outpatient, was determined. Further, the analyzed studies aimed to discern the absence of OUD preoperatively and its presence in the post-operative period; hence, the criteria used to ascertain OUD in the analyzed studies were also examined. Attempt was also made to delineate the route of opioid administration i.e. oral vs. parenteral, and whether the likely prescribers were anesthesiology, surgery, or primary care providers. In addition, whether the studies accounted for the persistent pain of surgery as a reason for the long-term opioid use was also discerned.
4. Results
The number of studies retrieved under each search term “perioperative”, “postoperative” and “preoperative” combined with terms “opioids” AND “addiction” were 87, 131 and 25 respectively (Figure 1). (After After accounting for the duplicate studies, which appeared under various search headings, 230 distinct articles were identified. The review of the abstracts and/or full texts yielded 17 articles, which evaluated the risk of OUD from opioids administered perioperatively and were therefore retained for further analysis (6-22). The excluded studies pertained to a range of topics including opioid sparing perioperative analgesic techniques, acute opioid tolerance, opioid induced hyperalgesia, perioperative use of ketamine, lidocaine and dexmedetomidine, and multimodal perioperative analgesia.
Adhering to the structure delineated in the introduction and the data extraction sections, the results are presented in the following order: (1) the phase of the perioperative opioid administration; (2) the criteria used to identify the OUD; (3) the study methodologies; (4) the populations of the surgical patients studied; and (5) the risk of new OUD.
Phase of the Perioperative Opioid Administration: The studies reviewed described the perioperative opioid administration mainly in terms of opioid prescriptions given for a variable period spanning the time of surgery (Table 1). In three studies, these were any opioid prescriptions given for postoperative pain within 6 to 90 days of the hospital discharge (6-8). In eight studies, opioid prescriptions filled within 2 to 4 weeks prior to, and 1 to 2 weeks after the surgery, were considered as perioperative opioids; the prescriptions given preoperatively were included presumably to allow for those filled before the surgery for the perioperative use (9-16). In six studies, only the pre and postoperative opioid prescriptions were noted, and the exact nature of perioperative opioids was not clear (17-22). The results, concerned primarily with the opioid prescriptions given for a variable period spanning the time of surgery, typically at the time of hospital discharge, therefore, highlighted mainly the outpatient phase of the perioperative opioid administration. Consequently, none of the analyzed studies distinctly evaluated the intraoperative, immediate postoperative recovery period, and the in-patient phases of perioperative opioid use. Although not always evident, the likely route of opioid administration was oral, and the prospective prescribers were surgical and/or primary care providers in most of the studies.
| Studies | Perioperative Opioid Use Described | Likely Phase of Perioperative Opioids | Diagnosis of Preoperative Opioid Use Disorder | Diagnosis of Postoperative Opioid Use Disorder |
|---|---|---|---|---|
| Alam et al. (6) | ≥ 1 opioid prescriptions within 7 days of hospital discharge | Oral opioid prescriptions at discharge | No opioid prescriptions within 1 year before surgery | ≥ 1 opioid prescription at 1 year from surgery (± 30 days) |
| Bateman et al. (9) | ≥ 1 opioid prescriptions the day of hospital discharge or within six days thereafter | Oral opioid prescriptions at discharge | Any outpatient prescriptions within 1 year before cesarean section and a diagnosis of opioid abuse excluded using ICD codes | Trajectory models - monthly patterns of opioid dispensing - patients with highest probability of filling opioids over time were defined as “persistent users” |
| Clarke et al. (7) | ≥ 1 opioid prescriptions 1 - 90 days after Surgery | Oral opioid prescriptions at discharge | No opioid prescriptions within 1 year before Sx including opioids and adjuvants | ≥ 1 opioid prescription 1 - 90 days and 91 to 180 days after surgery |
| Johnson et al. (11) | > 1 opioid prescription between 30 days before and 2 weeks after surgery | Oral opioid prescriptions at discharge | Patients did not fill an opioid prescription between 1 and 12 months before surgery and without a diagnosis of opioid dependence or abuse (ICD-9: 304.00-304.02 and 305.5-305.53) | Additional opioid prescription between 90 and 180 days after surgery |
| Brummett et al. (13) | > 1 opioid prescription between 30 days before and 2 weeks after surgery | Oral opioid prescriptions at discharge | No opioid prescriptions filled in 12 months to 31 days before surgery | Additional prescriptions filled between 90 - 180 days after surgery |
| Lee et al. (14) | > 1 opioid prescription between 30 days before and 2 weeks after surgery | Oral opioid prescriptions at discharge | No opioid prescriptions filled between 12 months and 31 days before surgery | ≥ 1 opioid prescription filled between 90 and 180 days after surgery. |
| Bennett et al. (17) | > 1 opioid prescription between 30 days before and 2 weeks after surgery | Oral opioid prescriptions at discharge | No opioid prescription fills in the 11 months prior to the perioperative period | Additional opioid prescription between 90 and 180 days after surgery |
| Harbaugh et al. (19) | > 1 opioid prescription between 30 days before and 2 weeks after surgery | Oral opioid prescriptions at discharge | No opioid prescription fills in the 11 months until 30 days prior to the surgery | ≥ 1 additional opioid prescription refill between 90 and 180 days after the surgical procedure |
| Swenson et al. (20) | > 1 opioid prescription between 30 days before and 2 weeks after surgery | Oral opioid prescriptions at discharge | No opioid fills for 8 months preceding, excluding the 30 days immediately prior | ≥ 2 opioid fills within 6 months of hysterectomy with ≥ 1 fill every 3 months and either total oral morphine equivalent ≥ 1150 or days supplied ≥ 39 |
| Bennett et al. (21) | > 1 opioid prescription between 30 days before and 2 weeks after surgery | Oral opioid prescriptions at discharge | No opioid prescription fills in the 11 months until 30 days prior to the surgery | ≥1 additional opioid prescription refill between 90 and 180 days after surgery |
| Olds et al. (22) | > 1 opioid prescription between 14 days prior to 7 days after surgery | Oral opioid prescriptions at discharge | No opioid prescriptions filled within 12 months to 14 days prior to surgery | Opioid prescription fills between 90 and 180 postoperative days and additional prescription fills 181 to 365 days |
| Goesling et al. (10) | Pre and postoperative opioid prescriptions | Oral opioid prescriptions at discharge | Patients who reported no opioid use the day of surgery | Opioid use reported by patients at 1, 3 months (phone), and 6 months (mail) |
| Sun et al. (12) | Pre and postoperative opioid prescriptions | Fentanyl patch and oral opioids- Likely opioid prescriptions at discharge | Not opioid prescriptions filled in the 12 months prior to surgery | ≥ 10 opioid prescriptions filled or > 120 day supply in the first year, after the first 90 days of surgery |
| Shah et al.(15) | Pre and postoperative opioid prescriptions | Oral opioid prescriptions at discharge | Diagnosis of opioid dependence and/or opioid overdose at surgery or at any preoperative encounter | A new diagnosis of opioid dependence and/or opioid overdose within 1 year post-operatively |
| Stark et al. (16) | Pre and postoperative opioid prescriptions | Oral opioid prescriptions at discharge | Patients using opioids on “daily basis” were excluded | “On-going” opioid use at 90 - 120 days |
| Hadlandsmyth et al. (18) | Pre and postoperative opioid prescriptions | Oral opioid prescriptions at discharge | Any outpatient prescription of non-injectable opioids | Continuously received opioids during the 12 months after TKA |
| Lindestrand et al. (8) | “95% received opioids during admission of which 84% were prescribed oxycodone” | Unclear- “81% had opioids prescribed at discharge” | No opioid prior to admission | Unclear- opioid prescriptions at 3 and 6 months |
Perioperative Opioid Use Described, Likely Phase and Criteria for Opioid Use Disorder
Criteria Used to Identify the OUD: The absence of preoperative OUD was based primarily on establishing the opioid naïve status, which was determined by varied criteria in the analyzed studies (Table 1). In four studies, the standard was no opioid prescriptions filled within the year prior to surgery (6-8, 18). In eight studies, the one-year exclusionary requirement disregarded prescriptions filled for a variable period immediately prior to the surgery-ranged from 30 to 14 days; ostensibly, to account for prescriptions filled preoperatively for the postoperative use (9-16). In one study, only the absence of diagnostic codes for “opioid dependence” or “opioid over dose” were considered (19). In four other studies the varied criteria used were: “no opioids used the day of surgery” (17), “no opioids used on daily basis” (20), “no outpatient prescriptions of non-injectable opioids” (21), and “no opioids prior to the admission” (22). The presence of new OUD after surgery, in most studies, rested on the “persistent opioid use”, which was based on variably recorded number of opioid prescriptions filled after the surgery (Table 1). In multiple studies, ≥ 1 opioid prescription filled between 3 to 6 months after the surgery constituted the persistent opioid use (8-13, 15-17, 20, 22). In two studies, the requirement was ≥ 1 opioid prescription filled at one year (6, 16). In one study, only the presence of new codes for “opioid dependence” and/or “opioid overdose” were used (19). In three other studies, the varied descriptions used for the new OUD after surgery included: the monthly patterns of opioid prescriptions filled (7), “≥ 2 opioid prescriptions filled within 6 months along with ≥ 1 prescriptions filled every 3 months and either total oral morphine equivalent dose of ≥ 1150 mg or ≥ 39 days of supply” (14), “≥ 10 opioid prescriptions filled in 1 year or > 120-day supply of opioids” (18).
Study Methodologies: The analyzed studies were not randomized or blinded, lacked comparative or placebo control groups, and were observational in nature (Table 2). In fifteen studies, the patients were selected retrospectively from a range of data sources, including health insurance claims, research databases, and electronic medical records, by using the various International Classification of Disease (ICD) and current procedural terminology (CPT) codes (6-16, 18, 19, 21, 22). In two studies, characterized as prospective and observational, the patient information was gathered by using self-reported questionnaires administered the day of surgery and by phone calls, e-mails and mail after the procedures (17, 20).
| Studies | Study Methodology | Study Population | Incidence of New Opioid Use Disorder |
|---|---|---|---|
| Alam et al. (6) | Retrospective data from health insurer database using CPT and ICD codes | Same day surgery (cataracts, TURP, varicose veins and laparoscopic cholecystectomy) | Persistent opioid use was 10.3% at 1 year in patients undergoing same day surgery procedures compared to 7.5% in those without similar exposure |
| Bateman et al. (9) | Retrospective data from health insurer database using CPT and ICD codes | Cesarean section | Persistent opioid use was 0.36% in women undergoing cesarean delivery |
| Clarke et al. (7) | Retrospective data from health insurer database using CPT and ICD codes | CABG, thoracotomy, thoracoscopy, laparotomy, laparoscopy, open and closed prostatectomy and hysterectomy | Persistent opioid use (> 90 days) after major elective surgeries was 3.1% |
| Johnson et al. (11) | Retrospective data from health insurer database using CPT and ICD codes | Common hand surgery procedures | Persistent opioid use (between 90 and 180 days) after common hand surgery was 13% |
| Brummett et al. (13) | Retrospective data from health insurer database using CPT and ICD codes | Common elective surgical procedures categorized as minor and major | Persistent opioid use ranged from 5.9% to 6.5% and it was similar in the 2 groups |
| Lee et al. (14) | Retrospective data from health insurer database using CPT and ICD codes | Curative-intent cancer surgery (lumpectomy, mastectomy, colectomy, pancreatectomy, esophagectomy, rectal, liver, gastric, and lung resection) | Persistent opioid use was 7% to 11% across the different surgeries |
| Bennett et al. (17) | Retrospective data from health insurer database using CPT and ICD codes | Cleft palate surgery in pediatric patients | Persistent opioid use was 4.4% following the cleft palate surgery in pediatric patients |
| Harbaugh et al. (19) | Retrospective data from health insurer database using CPT and ICD codes | Common surgeries in adolescents and young adults (tonsillectomy, adenoidectomy, inguinal, umbilical or epigastric hernia, appendectomy, cholecystectomy, pectus repair, colectomy, ORIF elbow, and arthroscopic ACL/meniscal repair, orchiopexy and hypospadias) | The overall persistent opioid use was 4.8%; it was 2.7% to 15.2% across the procedures, compared to 0.1% in the nonsurgical group |
| Swenson et al. (20) | Retrospective data from health insurer database using CPT and ICD codes | Hysterectomy | Persistent opioid use was 0.5% after hysterectomy |
| Bennett et al. (21) | Retrospective data from health insurer database using CPT and ICD codes | Bariatric procedures (abdominoplasty, panniculectomy, breast reduction, mastopexy, brachioplasty, thigh plasty) | Persistent opioid use was 6.1% after bariatric surgery procedures |
| Olds et al. (22) | Retrospective data from health insurer database using CPT and ICD codes | Plastic and reconstructive surgeries | Persistent opioid use was 6.6% and prolonged opioid use was 2.3% in patients undergoing plastic and reconstructive surgeries |
| Goesling et al. (10) | Prospective data collected using self-reported questionnaires, phone calls and mail | Total knee and hip arthroplasty | Persistent opioid use at 6 months was 8.2% for knee and 4.3% fin hip arthroplasty patients |
| Sun et al. (12) | Retrospective data from health insurer database using CPT and ICD codes | TKA, THA, laparoscopic and open cholecystectomy, appendectomy, cesarean, FESS, cataract, TURP, mastectomy | Persistent opioid use ranged from 1.28% for cesarean section to 5.10% for TKA |
| Shah et al.(15) | Retrospective data from health insurer database using CPT and ICD codes | Urological surgeries | 0.09% of patients undergoing urological surgery were diagnosed with a new diagnosis of opioid dependence or overdose |
| Stark et al. (16) | Prospective data collected using self-reported questionnaires, phone calls and mail | All surgeries except cancer related and minor procedures | The overall persistent opioid use (> 90 days) was 10.5%; it was 23.6% after spinal and 13.7% after orthopedic surgery procedures |
| Hadlandsmyth et al. (18) | Retrospective data from health insurer database using CPT and ICD codes | Knee arthroplasty | Persistent opioid use in TKA patients was 12% at 3 months, 4% at 6 months, and 2% at 12 months |
| Lindestrand et al. (8) | Retrospective data from health insurer database using CPT and ICD codes | Surgery for hip Fractures | Persistent opioid use was 2.9% at 6 months in patients undergoing surgery for hip fracture |
Study Methodologies, Study Populations and Incidence of New Opioid Use Disorder
Populations of Surgical Patients Studied: The populations of surgical patients studied varied from a narrow scope such as those undergoing cesarean delivery (7), hand surgery (9), cleft palate surgery (12), hysterectomy (14), hip and knee surgeries (17, 21, 22), urological surgeries (19), to much broader range such as those undergoing curative-intent surgery for cancers (11), bariatric surgeries (15), plastic and reconstructive surgeries (16), same day surgeries (6), surgeries in adolescents and young adults (13), “major elective” surgeries (8, 20) and “common major and minor” surgeries (Table 2) (10, 18).
Incidence of New OUD: With only the observational studies employing, variable methodologies, non-standardized outcome criteria, and heterogeneous study populations, the results could not be pooled. Though the overall risk of new OUD from the perioperative opioids, as defined in these studies, increased, it varied significantly from as low as 0.09% to as high as 13%, (Table 2) (9, 19). The highest rates of opioid misuse were reported in subsets of patients undergoing spinal (23.6%) and orthopedic surgeries (13.7%) (20). When the most frequently used methodology of retrospective data collection from the health insurers was employed, the results reported also varied; lowest rates were reported after cesarean section (0.36%) (7) and hysterectomy (0.5%) (14), one study reported no difference in the rates amongst “minor” (5.9%) and “major” (6.5%) surgeries (10), while another reported wide ranging results (2.7% to 15.2%) in patients undergoing “common” surgical procedures (13).
Significant Limitations of the Analyzed Studies: In addition to the retrospective and observational nature, and evaluation limited to the outpatient phase, the analyzed studies had significant other methodological limitations. In multiple studies, as detailed above, the sporadically filled opioid prescriptions, in a defined period, constituted the opioid misuse. Yet, the accepted criteria for OUD emphasize the compulsive use of opioids despite physical, mental, and social harm over a course of 12-month period (Box 1) (23). Consequently, the accepted standards for ascertaining OUD were not met, both pre and postoperatively, in most studies. Furthermore, opioid prescriptions filled, or given, were used as a proxy for the opioid use by the patients in most studies. However, this criterion could not account for opioids obtained from other sources (e.g. family, friends, illegal suppliers), unfilled prescriptions, and the diverted drugs, potentially confounding the results. Moreover, the persistent opioid use, denoting new OUD after surgery, could be explained by the continuing pain of surgery in many studies (7, 13, 14, 20). Also, attempts to exclude conditions predisposing to substance use disorders in many studies such as illegal drug use, other prescription medication misuse, and specific psychiatric disorders, were prone to underreporting of these conditions. The studies centered on insurance utilization claims were additionally restricted by evaluation of only specific patient groups and the remotely collected patient information, based on the codes, was susceptible to multiple recording inaccuracies.
| A Problematic Pattern of Opioid Use Leading to Clinically Significant Impairment or Distress As Manifested by at Least Two of the Following Occurring Within 12-Month Period (23). |
|---|
| 1. Opioids taken in larger amounts or over longer period than intended. |
| 2. A persistent desire or unsuccessful effort to cut down or control opioid use. |
| 3. A great deal of time spent in activities necessary to obtain the opioid, use the opioid, or recover from their effects. |
| 4. Craving or a strong desire or urge to use opioids. |
| 5. Recurrent opioid use resulting in a failure to fulfill major role obligations at work, school or home. |
| 6. Continued opioid use despite persistent or recurrent social or interpersonal problems caused or exacerbated by the effects of opioids. |
| 7. Important social, occupational, or recreational activities are given up or reduced because of the opioid use. |
| 8. Recurrent opioid use in situations in which it is physically hazardous. |
| 9. Continued opioid use despite knowledge of having a persistent or recurrent physical or psychological problem that is likely to have been caused or exacerbated by the opioids. |
Diagnostic and Statistical Manual-5 Criteria for Opioid Use Disorder
5. Discussion
After review of the literature it is apparent that the compelling issue of the risk of OUD from opioids given in the perioperative period has not been adequately assessed. With no available data, the precise risk of OUD from opioids given in the intraoperative, immediate post-operative recovery and in-house phases of perioperative period remains unknown and unevaluated. Consequently, no clear recommendations for averting OUD from opioids given during intraoperative and postoperative recovery periods can be extended to the anesthesia providers. Furthermore, the analyzed literature, constrained mainly to the outpatient phase of perioperative care, has substantial methodological limitations, rendering the results deficient. Therefore, the available recommendations for outpatient use of opioids after surgery (24), are also based on insufficient evidence. Consequently, to formulate any putative recommendations against this backdrop, the necessity and distinct use of opioids by the various providers, and the differential risk of OUD at the various perioperative stages, must first be examined.
Distinct use of Opioids by the Heterogeneous Providers: The diverse providers of perioperative care, involved at specific phases of perioperative period, use opioids distinctively. Intraoperatively and in the immediate postoperative recovery period, the patients are managed mainly by anesthesia providers, and the opioids are valuable adjunct to the care provided. Opioids are key to balanced anesthesia care, which relies on synergistic effects of multiple drugs in order to limit adverse reactions from a single agent (25). By blunting the autonomic nervous system instability, the opioids limit the hemodynamic variations caused by the surgical trauma. These properties are useful, especially, in the critically ill patients and in those with diminished cardiopulmonary reserves (25). Similarly, due to their potent analgesic properties, opioids are routinely used in the immediate post-operative recovery period. Opioids are also regularly prescribed, generally as prescriptions for oral use, for outpatient analgesia after surgery. Typically, a wide range of surgical providers initiate these prescriptions, which are continued variably by surgeons, primary care providers, internists, and pain specialists.
Incongruent Drivers of Opioid Misuse: The disparate drivers of OUD in the perioperative period are at-play incongruently throughout the various perioperative stages (Box 2). Addressed only cursorily in the literature, the pertinent variables can include: patient’s level of consciousness, their ability to perceive the euphoric opioid effects, and the severity of pain experienced, at the time of opioid administration (4). In the context of perioperatively care the effects of hypnotic and sedative drugs administered intraoperatively, and the intensity and duration of the postoperative pain experienced, both immediately after the surgery and long-term, are noteworthy (4). Furthermore, patient’s capacity to, either directly or indirectly, influence the opioid use is relevant (5). This ability is absent under anesthesia, limited during postoperative recovery period, and substantial during the out-patient stage (4). Similarly, the level of surveillance and monitoring of the patient’s opioid use is vital (5). The latter is stringent during the intraoperative and recovery phases, and variable and limited during the out-patient stage (4). The duration of opioid exposure is directly linked to the potential for misuse (5), and it can vary extensively from minutes for the intraoperative to months for the out-patient phase (4). Correspondingly, prescribers experience and knowledge of the risk of OUD is critical, as the incongruity of the providers would augment the potential for erroneous prescribing practices and the subsequent risk of misuse (5). The perioperative providers are chiefly analogous during the intraoperative and recovery periods but are variable and disparate during the in-house and outpatient stages.
| Drivers of Opioid Misuse |
|---|
| 1. Level of consciousness at the time of opioid administration |
| 2. Ability of patients to perceive the opioid effects |
| 3. Influence of other concurrently administered drugs e.g. hypnotic and sedative agents |
| 4. Severity of the pain experienced at the time of opioid administration |
| 5. Ability of patients to directly or indirectly control their opioid use |
| 6. Patients’ discretion in determining the use of opioids provided |
| 7. Whether the drugs are administered directly by the providers or are consumed by the patients |
| 8. Level of patient monitoring |
| 9. Surveillance of patients’ opioid use |
| 10. Period of opioid administration-varying from minutes to months in the perioperative period |
| 11. Expected duration of pain-varying from days to months in the perioperative period |
| 12. Type, route and potency of the opioids administered e.g. ultra short-acting intravenous vs. |
| 13. Extended release oral preparations |
| 14. Heterogeneity of prescribers e.g. anesthesiologists vs. a range of surgeons, primary care providers, pain specialists, and internists |
| 15. Prescribers’ background, experience and knowledge of prescribing opioids |
Possible Risk of OUD at Various Perioperative Stages: In terms of the risk of misuse, the perioperative period can be categorized into four discrete phases (Table 3). Intraoperatively, a consistent group of practitioners (anesthesia providers) directly administer the drugs, under strictly controlled and monitored conditions, for a relatively brief and well-defined period. Opioids are given alongside powerful hypnotic and sedative drugs and the patients, unconscious or heavily sedated, are generally unable to recall their euphoric effects or direct their use (4). Therefore, the risk of OUD from intraoperative opioids, though unknown, is likely minimal (4, 5). In the immediate postoperative recovery period, the patients, while recovering from the effects of anesthesia and surgery, can be stupors and in significant pain. Though opioids are administered under strictly controlled conditions, and for a brief and finite period, the patients can experience their euphoric effects and to an extent may control their use (4). Hence, the risk of OUD from opioids given at this stage, though also unknown, and possibly greater than intraoperative stage, may still be low (4, 5). The inpatient stage of opioids use can be characterized by opioids of different types, and potency, given by a range of incongruent providers including surgeons, hospitalists, anesthesiologists, and acute pain specialists. The patients can often regulate their opioid use and can generally experience the euphoric effects. Although the drugs are given for a well-defined period, and under close supervision, the multiple conflicting variables engender potential for abuse which is difficult to ascertain. Opioids for out-patient use after surgery are predominantly prescriptions for oral consumption provided by a broad range of prescribers discussed earlier. The duration of opioid use can vary significantly from days to months (e.g. days after arthroscopy to months after spinal fusion), during which the opioid use is monitored variably, and only indirectly. Moreover, the patients, cognizant of the euphoric effects, can fill the prescriptions, and use the drugs, almost autonomously, and opioid prescriptions for outpatient analgesia are known for diversion and misuse (3, 5). Furthermore, the available literature indicates that the quantity of opioids prescribed for out-patient use after surgery is often in excess of the patient’s analgesic requirements and are prone to misuse (2, 3). Overall, contrary to intraoperative and immediate recovery periods, the potential for misuse from opioid given for outpatient analgesia after surgery, though not precisely ascertained, can be significant.
| Intraoperative | Postoperative Recovery | Inpatient | Outpatient | |
|---|---|---|---|---|
| Likely opioid prescribers | Anesthesiologists and CRNAs | Anesthesiologists, CRNAs, and RNs | Various surgeons, internists, anesthesiologists, pain teams, PAs, RNs | Range of surgeons, interventionists, primary care providers, pain specialists, and PAs |
| Training, experience and heterogeneity of the Prescriber | Routinely prescribe, specific training, and uniform | Routinely prescribe, specific training, and uniform | Variable training and experience, and heterogeneous | Variable training and experience, and highly heterogeneous |
| Concomitantly administered drugs | Hypnotics, sedatives and other adjunct analgesics | Adjunct analgesics, regional and neuraxial anesthesia | Adjunct analgesics, regional and neuraxial anesthesia | Highly variable |
| Level of consciousness | Heavy sedated or unconscious | Possibly sedated and stupors | Unlikely sedated | Generally awake and alert |
| Awareness of opioid euphoric experience | Unlikely | Partial | Intact | Intact |
| Level of pain experienced | Autonomic instability | Significant | Variable | Variable |
| Patient control of opioid use | None | Minimal | Significant | Substantial |
| Administration of opioids | Direct administration by the providers | Direct administration by the providers | Given under close supervision | Consumed by the patients themselves |
| Type of opioids prescribed | Potent, short acting | Potent, short acting | Variable | Variable |
| Route of administration | Intravenous | Generally intravenous | Intravenous, oral | Generally oral, transcutaneous |
| Duration of administration | Finite, short-minutes to hours | Finite, short-minutes to hours | Finite, variable-days | Long, highly variable-days to years |
| Monitoring of patient’s | Extensive-ASA standards | Extensive | Variable but direct | Minimal and indirect |
| Monitoring of patients’ opioid use | Direct | Direct | Variable but direct | Minimal and indirect |
| Primary decision maker of the opioids used | Anesthesia providers | Anesthesia providers | Heterogeneous | Heterogeneous but ultimately patients themselves |
| Potential for OUD | Unknown but unlikely | Unknown but less likely | Unknown | Unknown but likely |
Phases of Perioperative Opioid Use in Terms of OUD
Putative Recommendation for Perioperative Opioid Use: With the unknown risk of OUD from opioids given in the intraoperative, postoperative recovery, and in-house stages and the inadequately defined risk from their outpatient use, recommendations for the opioid use in perioperative period can be based on: (1) the likely risk of opioid misuse; (2) the necessity of opioid use; (3) adverse reactions from the opioids used; (4) adverse reactions from the opioid alternatives. Intraoperatively: the likely risk of OUD is minimal, opioids are central to smooth anesthesia, and the potential for adverse reactions from opioid alternatives can be significant, especially in the critically ill patients (26). Consequently, careful intraoperative use of opioids, particularly in the aforementioned subset of patients, at dosages averting acute opioid tolerance (27), may be prudent. Similarly, the likely lower risk of OUD in the immediate postoperative recovery period, alongside the well-known opioid adverse effects and the adverse reactions from alternative analgesic strategies, should be considered prior to withholding opioids for the concerns of misuse. With literature broadly suggestive of high risk of OUD from prescription opioids for outpatient use (2, 3, 5) it may be useful to heed the proposed recommendations for outpatient analgesia after surgery, despite their unsubstantiated disposition. The latter generally endorse opioids of appropriate type and quantity prescribed commensurate to the nature of surgery and the expected duration of post-surgical pain, close monitoring of the patients’ opioid consumption, and the preferential use of non-opioid analgesics (24). Following this pattern, weaning patients off opioids, and employing alternative non-opioid therapies prior to the hospital discharge after surgery, may be advantageous. Overall, in view of the substantial risks posed by the OUD, and the utility of perioperative opioids, substantive recommendations can only be offered if the risk of OUD is clearly delineated for the various perioperative stages, while avoiding the limitations noted in this review.
6. Conclusions
Disparity in drivers for misuse in the perioperative period could results in dissimilar potential for OUD from opioids given at the various phases of perioperative care. Systematic search and analysis of the pertinent literature showed that the analyzed studies have notable methodological limitations and that the evaluation is limited to the out-patient phase of perioperative period. Consequently, the links between OUD and the opioids given perioperatively have not been adequately accessed. Though proposal can be formulated, substantive recommendations would require clear delineation of the risk of OUD from opioids given at various stages of perioperative care, while avoiding the limitations noted in this review.