1. Background
Usually, optimizing the cardiac preload or determining the requirement for intravenous (IV) fluid is the first step in treating patients with unstable hemodynamic (1). It should be noted that excessive or unnecessary fluid administration could negatively affect the patients’ hemodynamic (2, 3). Classically central venous pressure (CVP) has been used to determine the need for volume expansion during past decades. However clinical symptoms (e.g., cold and pale skin, increased heart and respiratory rate, low urinary output, and weak arterial pulse) have the highest diagnostic value. In a person with normal heart and lung, the CVP, and right and left heart’s filling pressures should have the same value. In pathologic conditions, such as left ventricular (LV) dysfunction or increased pulmonary vascular resistance (PVR), the correlation between these pressures may be destroyed, and CVP ability to estimate the LV filling pressure limited (4). In such conditions, the insertion of a pulmonary artery catheter (PAC) and measuring the pulmonary artery occlusion pressure (PAOP) are useful to estimate the LV filling pressure (5, 6). In recent decades, it has been demonstrated that both CVP and PAOP have relatively high misleading values, particularly in critically ill patients. Thus some new modalities has been presented (6, 7). Respiratory induced hemodynamic changes and transesophageal echocardiography have been used successfully in this way. Considering CVP and PAOP as static indices, respiratory induced hemodynamic changes are called “dynamic indices”. In a person with normal body status, throughout spontaneous respiration, the hemodynamic changes during mechanical ventilation are not significant, but in some pathological conditions (e.g., hypovolemia, asthmatic status, pneumothorax), respiration may deeply affect the patient’s hemodynamic. In anesthetized patients under full ventilatory support, with increased ventilatory induced hemodynamic changes (VIHG), it is logical to search for pathological conditions such as hypovolemia (8, 9). These hemodynamic changes reflected in stroke volume (SV), cardiac output (CO), systolic (SBP), diastolic (DBP), mean arterial blood pressures (MAP), and pulse pressure (1). In clinical practice, the rate of these hemodynamic changes can easily be calculated during invasive hemodynamic monitoring. We theorized that other pathological conditions may affect the severity of hemodynamic changes, those should be considered for all ICU patients (9-11).
2. Objectives
Thus in this cross-sectional investigation, we measured the VIHG and studied the correlation between the severity of VIHG and any other co-existing data following the cardiac surgery.
3. Methods
This study was conducted in the post-cardiac surgery ICU of Madani teaching hospital (Tabriz-Iran) on adult patients who were admitted to the ICU while were anesthetized and required mechanical ventilation. The study was approved by the local institutional ethical committee (registration number: 1395.732). Participants were patients who underwent elective cardiac surgery without any intervention on the treatment or monitoring activities. Patients aged under 18 years old, redo or urgent surgery, preoperative renal, neurologic and pulmonary disease, diabetes mellitus, and participants of any other clinical trials were excluded from the study. In a nine-month period (August 2017 to April 2018), 304 patients were enrolled in the study, that all of them had an indwelling arterial (radial) and central venous catheter. After admitting to ICU, all data due to the patient’s demography, anesthesia, and surgery were explored and recorded. Using frozen display at an appropriate phase-in Grid texture, the systolic, diastolic, and pulse pressure variations due to respiratory phases were calculated, every hour up to three-hour. Patients with following criteria during the first 3-hour period were excluded from the study: spontaneous breathing or irregular cardiac rhythm (e.g., atrial fibrillation), ventilation with tidal volume ≥ 10 mL/kg, PEEP ≥ 10 cmH2O, lack of access to the patient (i.e., re-admitting to the operating room or Cat lab), and invasive monitoring failure.
In the first 24-hour of the ICU stay, the following data were explored and recorded: the presence of open sternum, redo surgery or re-opening of the sternum, diagnosis of cardiac tamponade, the amount of blood-shedding, hypotension episode, anuria/oliguria (urinary output < 0.5 mL/kg/h), inotropic, vasopressor and vasodilator usage, and blood products requirement. The following data were also collected: the time of ventilatory support, duration of ICU stay, and cardiac, respiratory, renal, neurologic, and infective complications. Cardiac function was calcified using the left and right ventricular ejection fraction (LVEF and RVEF) in two subgroups (≥ 0.4 and < 0.4).
3.2. Statistical Analysis
Data were analyzed using SPSS version 23.0. MAP was calculated by
4. Results
At the end of the study, out of 304 patients who enrolled in the study, 292 met the inclusion criteria, that most of them were male (64.4%). The mean age, weight, and height were 51.5 ± 16.70 years, 65.04 ± 8.56 kg, and 168.36 ± 8.96 cm, respectively. Coronary artery bypass grafting (CABG) was the most common surgery (64.4 %) (Table 1).
| Values | |
|---|---|
| Male/Female | 188/104 |
| Weight, kg | 65.04 ± 8.56 |
| Height, cm | 168.36 ± 8.96 |
| Age, y | 51.50 ± 16.70 |
| Type of surgery | |
| CABG | 188 (64.4) |
| VHD | 50 (17.1) |
| CABG/VHD | 25 (8.6) |
| ASD/VSD closure | 15 (5.1) |
| Bentall operation | 6 (2.1) |
| Mass resection | 4 (1.4) |
| Others | 4 (1.4) |
Abbreviations: ASD, atrial septal defect; CABG, coronary artery bypass grafting; VHD, valvular heart disease; VSD, ventricular septal defect.
aValues are expressed as mean ± SD or No. (%).
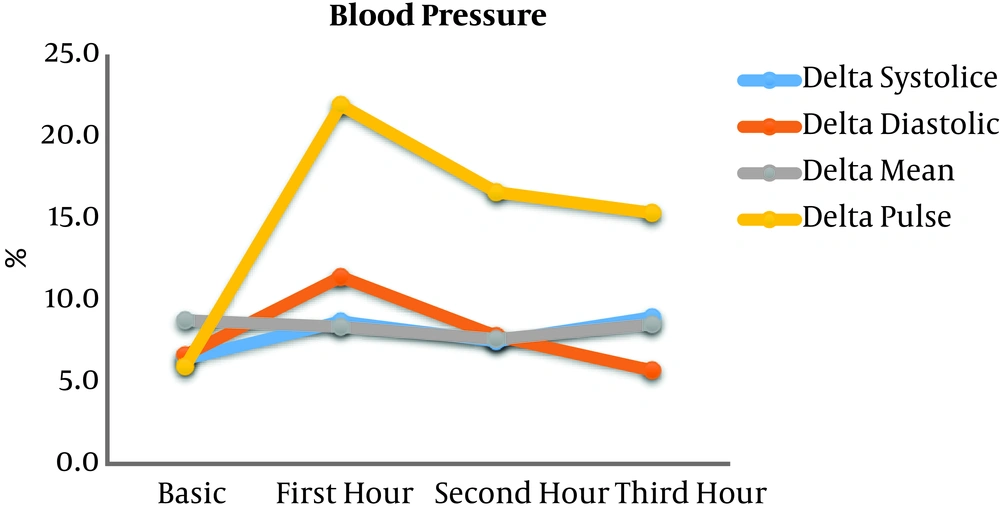
Anesthesia time was 272.60 ± 54.01 min. Cardiopulmonary bypass (CPB) was used in 95.55% of the surgeries, with CPB time 97.47 ± 26.18 min. The left (LV) and right (RV) ventricular ejection fraction and pulmonary artery pressure (PAP) are presented in Table 2. These data were extracted from pre- and intra-operative echocardiographic and invasive measurements. When data on echocardiographic reports were reported qualitatively, the “normal” LVEF, RVEF, and PAP values considered as 0.55, 0.55, and 20 mmHg, respectively. When the word “preserved” had been used, the LVEF and RVEF were considered as 0.40 and 0.40, respectively. In the absence of any data, the LVEF, RVEF, and PAP values considered as 0.50, 0.50, and 20 mmHg, respectively. Because of the returning of the spontaneous breathing in most of the patients during the following hours, the calculation of the VIHG was done only in the first 3 ICU-hour (Figure 1). As illustrated, the ΔPP had the most intense variation, thus we used its mean value to find any correlation with other factors or outcomes. In this way, we classified patients based on the ΔPP severity in two subgroups: ≤20% vs. >20% (table 2). As shown in the table, in cardiac dysfunction (ejection fraction < 0.4), the ΔPP was more increased during mechanical ventilation.
| ΔPP, % | Total | P Value | ||
|---|---|---|---|---|
| ≤ 20 | > 20 | |||
| Anesthesia time, min | 269.67 ± 55.626 | 280.64 ± 49.052 | 272.60 ± 54.01 | 0.13 |
| CPB time, min | 95.69 ± 26.532 | 102.49 ± 24.637 | 97.47 ± 26.18 | 0.07 |
| Preoperative LVEF, % | 44.57 ± 9.711 | 30.42 ± 7.096 | 40.79 ± 11.03 | 0.01b |
| Preoperative RVEF, % | 45.34 ± 5.963 | 42.44 ± 8.996 | 44.59 ± 7.007 | 0.02b |
| Preoperative PAP, mmHg | 22.75 ± 6.198 | 22.87 ± 5.421 | 22.78 ± 5.991 | 0.88 |
| Basic MBP, mmHg | 100.63 ± 15.088 | 94.03 ± 14.525 | 95.80 ± 14.94 | 0.77 |
| Basic CVP, mmHg | 8.14 ± 3.938 | 7.31 ± 3.543 | 7.92 ± 3.848 | 0.08 |
Abbreviations: CPB, Cardiopulmonary bypass, CVP, central venous pressure; LVEF, left ventricular ejection fraction; MBP, mean blood pressure; ΔPP, pulse pressure variation; RVEF, right ventricular ejection fraction.
aValues are expressed as mean ± SD.
bP value < 0.05.
Data collected during the ICU stay and the first 24-hour period are shown in Table 3. Mortality and morbidity occurred in 2 (0.68%) and 50 (17.12%) patients, respectively. Fifty-one patients underwent re-operation during the first 24-hour: 19 patients for sternum closure, 18 patients for bleeding control, 12 patients because of cardiac tamponade, and 2 patients for coronary artery graft revision. Nine patients had oliguria/anuria episodes in this period, with an increased chance of renal dysfunction in the following days (odd ratio 3.2; 95% CI: 1.58 to 5.92; P = 0.01). The bleeding rate during the first 24-hour was 1052 ± 549 ml (200 - 4000 ml) and in this period, fluid balance was -525 ± 724 mL (-1900 - 2000 mL).
Finally, we compared patients with ΔPP ≤ 20% and those with ΔPP > 20%. Patients with LVEF or RVEF of < 0.4 had more ΔPP than patients with ≥ o.4 value (Table 3). In the same way, the hemorrhage rate and fluid balance in the first 24-hour period were classified in various subgroups; there was no significant correlation between hemorrhage rate or fluid balance and mean ΔPP (P value > 0.05). Comparing to patients who underwent emergency surgery because of any reason except for cardiac tamponade (sternum closing or bleeding control surgery), patients who underwent emergency surgery because of cardiac tamponade had an increased ΔPP. Comparing to patients with a closed sternum, an open sternum did not have any significant effect on ΔPP. Requirements to inotropic, vasopressor and vasodilator agents, and blood products did not have any correlation with ΔPP. Mortality and complications rate, time to tracheal extubation, and ICU stay did not have any correlation with ΔPP severity.
| ΔPP | Total | P Value | ||
|---|---|---|---|---|
| ≤ 20% | > 20% | |||
| Left ventricular dysfunction (yes/no) | 73/141 | 74/4 | 147/145 | 0.001b |
| Closed sternum/unclosed sternum | 27/187 | 13/65 | 40/252 | 0.37 |
| Redo operation during the first 24-hour (yes/no) | ||||
| Tamponade | 1/213 | 10/68 | 11/281 | 0.04b |
| Bleeding control | 15/199 | 3/75 | 18/274 | 0.32 |
| Sternum closure | 11/203 | 8/70 | 19/273 | 0.12 |
| Redo CABG | 1/213 | 1/77 | 2/290 | 0.46 |
| Total | 30/154 | 21/57 | 51/241 | 0.01b |
| Inotrope usage during the first 24-hour (yes/no) | 103/111 | 46/32 | 149/143 | 0.10 |
| Vasopressor usage during the first 24-hour (yes/no) | 98/116 | 49/29 | 147/145 | 0.51 |
| Vasodilator usage during the first 24-hour (yes/no) | 100/114 | 40/38 | 140/152 | 0.49 |
| PC usage during first the 24-hour (yes/no) | 127/87 | 43/35 | 170/122 | 0.06 |
| Platelet usage during the first 24-hour (yes/no) | 69/145 | 21/57 | 90/202 | 0.38 |
| FFP usage during the first 24-hour (yes/no) | 123/91 | 41/37 | 164/128 | 0.45 |
| Mortality (yes/no) | 0/214 | 2/76 | 2/290 | 0.071 |
| Major post operative complication (yes/no) | ||||
| Cardiac | 13/201 | 2/76 | 15/277 | 0.37 |
| Renal | 16/198 | 6/72 | 22/270 | 0.56 |
| Neurological | 4/210 | 1/77 | 5/287 | 0.59 |
| Respiratory | 13/201 | 7/71 | 20/272 | 0.43 |
| Others | 3/211 | 1/77 | 4/288 | 0.71 |
| Total | 35/179 | 15/63 | 50/242 | 0.33 |
| Ventilatory support, h | 12.97 ± 9.77 | 14.55 ± 13.91 | 13.4 ± 11.0 | 0.54 |
| ICU stay, d | 4.31 ± 1.61 | 4.55 ± 2.17 | 4.4 ± 1.8 | 0.153 |
Abbreviations: CABG, coronary artery bypass grafting; FFP, fresh frozen; PC, packed cell plasma.
aValues are expressed as mean ± SD.
bP value < 0.05.
Ventilatory parameters and their correlation with ΔPP intensity were also investigated. We compared the PEEP ≤ 5 vs. > 5 cmH2O, inspiratory pause fraction ≤ 0.3vs. > 0.3, and airway plateau pressure ≤ 25 vs. > 25 cmH2O. The ΔPP was not different.
5. Discussion
In the treatment of patients with unstable hemodynamics, fluid expansion is the first step (1-3). Static or hemodynamic parameters are used to guide the correct decision (4-6). But caution should be taken, because some factors may be misleading (6). It is believed that the new hemodynamic indices (VIHG) are more clinically valuable in deciding to administer additional fluid (8-13). Marik et al. (9) in a systematic review investigated the ability of dynamic versus static indices in determining the hypovolemia. They concluded that in critically ill patients, VIHG had more accuracy than traditional static indices of volume responsiveness (9).
The current study, that was conducted on adult patients who had cardiac surgery, investigated the ventilatory induced hemodynamic variations (mainly ΔPP) and factors affecting those. To achieve this goal, we classified the patients into two subgroups; ≤ 20% and > 20%. To get an exact conclusion, those who were simultaneously participating in another trial were excluded (14). We studied the VIHG only at the first 3-hour period due to the spontaneous breathing return. Although it is believed that exaggerated VIHG is mainly related to the hypovolemic states (15) but some other pathological conditions (e.g., cardiac arrhythmias, pulmonary diseases, or opened sternum) may obscure this relation (16). In the current study, the ΔPP had the highest significant value. Thus we used it as the main VIHG. Kubitz et al. (17) in a study on anesthetized and mechanically ventilated pigs reported the same results; that ΔPP is superior to ΔSBP in guiding fluid therapy. On the contrary, some authors stated that ΔPP is not a valid predictor of fluid requirement in patients with LV failure (18). In the current study, cardiac dysfunction and tamponade were the most important cause of increased ΔPP. Carmona et al. (19) discussed the same pathophysiology in their investigation. Kronas et al. (12) in an experimental animal study showed that after inducing acute myocardial damage, ΔSBP and ΔPP didn’t reflect volume responsiveness. We believe that in post-cardiac surgery ICU, any increase in VIHV must trigger to suspect cardiac dysfunction and tamponade; such an approach may save some patients. It may be concluded that for all patients with an increased VIHV, when volume expansion is not curative, we must consider cardiac dysfunction and tamponade as the casuals. Another finding of the present study was the maintenance of VIHV validity in patients with the open sternum. Although many studies have questioned its validity in the unclosed sternum (20), some reports supported its validity for patients who had open chest surgery (21). Also, administrating inotropic or vasopressor agents may delay the diagnosing of hypovolemia, hence it can be concluded that these drugs do not affect VIHV validity.
Lack of correlation between fluid balance and VIHG may support the hypothesis that the fluid balance of critically ill patients should be kept positive (22). However, van Mourik et al. (23) concluded that a positive fluid balance in acute respiratory patients is associated with an increased risk of mortality and morbidity. In the current study, the complication rate, the required time to tracheal extubation, and the duration of the ICU stay were the same in two ΔPP subgroups. However, it must be remembered that any delay in diagnosis and management (logically) will affect the patient’s outcomes. All of our patients were ventilated with ordinary parameters. Thus their ΔPPs were the same.
5.1. Conclusions
In the current study, the ΔPP was the most sensitive dynamic index. Cardiac dysfunction and tamponade increased ΔPP, thus it may be concluded that in the post-cardiac surgery ICU, when the patient is not volume responsive, the clinician must consider cardiac dysfunction or tamponade. Unclosed sternum does not affect ΔPP validity. ΔPP value does not affect post-op complications rate, time to tracheal extubation, or ICU stay.