1. Context
The immune system has evolved to protect the body through developing appropriate immune responses against potentially harmful pathogens and non-compliant antigens. The immune system uses several mechanisms to regulate these responses which may lead to tumor growth, rejection of organ transplantation, autoimmune diseases, asthma, and allergies. The prevalence of diseases related to immune regulation has increased significantly in recent decades (1, 2).
The immune response is the response to microbial components and macromolecules such as proteins, polysaccharides, and small chemicals that are identified as foreigners, regardless of their physiological and pathological consequences. The body’s defense system against germs contains two parts, the innate immunity (which comes into play immediately after identifying the pathogen) and the acquired immune responses. Innate immunity response includes cellular and biochemical defense mechanisms, such as epithelium and antimicrobial substances released by it, neutrophils, macrophages, natural killer (NK) cells, blood proteins, and other inflammatory mediators that exist even before the infection (3). The acquired immune response usually occurs two to three days after exposure to antigens and its severity increases after each recurrence. Lymphocytes and their secreted products are the main components of the acquired immunity. T cells play a vital role in humoral and cellular responses through direct contact and cytokines release (4-6). Cytokines are key modulators of inflammation that play both inflammatory and anti-inflammatory roles. Cytokines contribute to the removal of antigens and play a key role in the drugs and systemic inflammatory responses (7, 8).
There is a constant balance between pro-inflammatory and anti-inflammatory cytokines (9). Following excessive pro-inflammatory cytokines production, inflammation is excessive, and the body cannot adapt to these conditions. Therefore, anti-inflammatory cytokines are vital in the process of inflammation and play an important role in both enhancing and suppressing inflammatory responses (3). It is reported that general anesthesia affects the immune system (10-12).
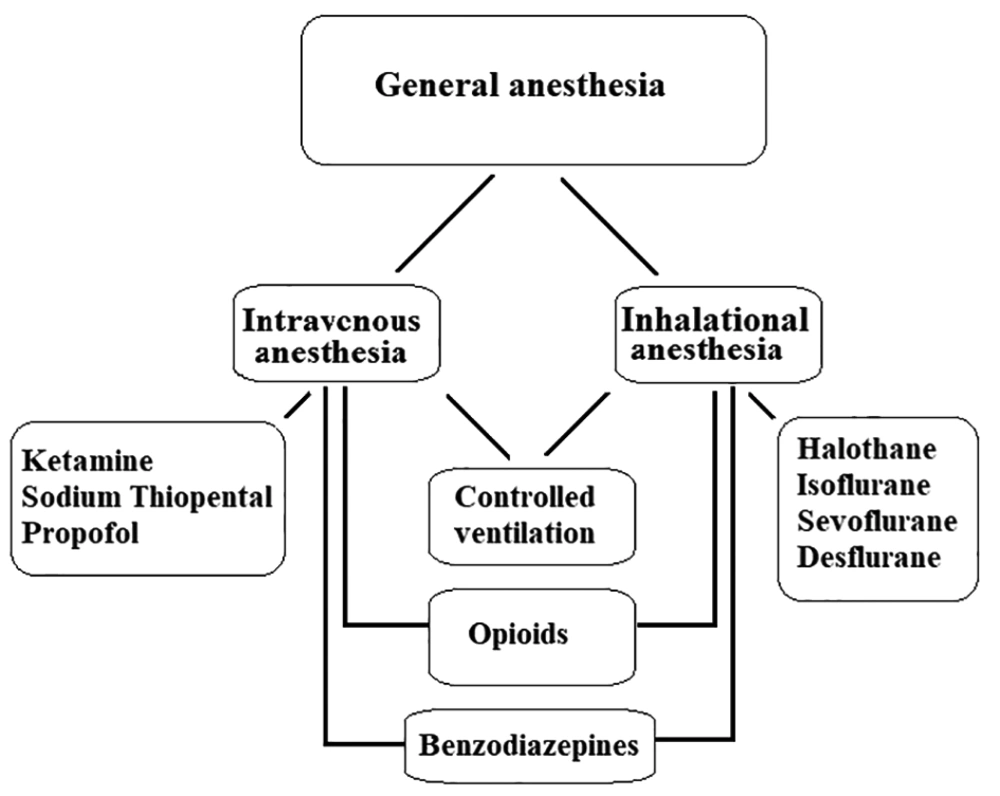
General anesthesia, which is a drug-induced deliberated state, is the most commonly used strategy in anesthesia care (13). There are various drugs to ensure unconsciousness, analgesia, amnesia, and loss of reflexes of the autonomic nervous system (14). General anesthesia usually induces using intravenous anesthetics, inhalational (volatile) anesthetics or a combination of both. Also, opioids and benzodiazepines are often used as adjuvants in general anesthesia. In some cases, ventilation of the patients should be controlled (Figure 1).
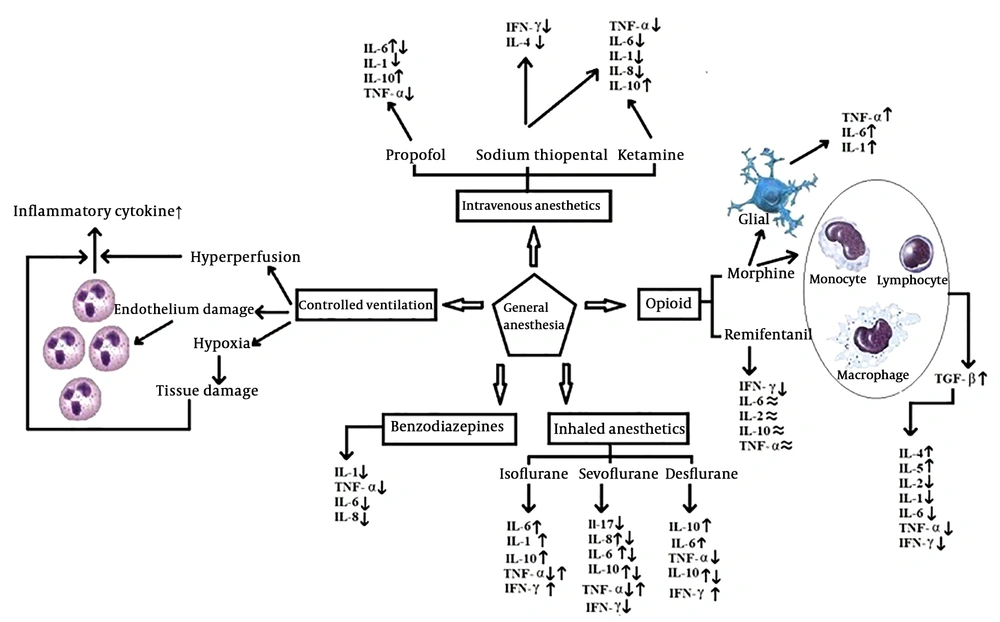
Anesthesia and drugs that administer to induce the anesthesia, affect the immune system, mainly immune cells, during the perioperative period. The effects of anesthesia on B-lymphocytes, T-lymphocytes, NK cells, macrophages, erythrocytes, and leukocytes is well documented (2). Anesthesia affects the pro-inflammatory and anti-inflammatory cytokines’ secretion. The main Pro-inflammatory cytokines include IL-6, Il-8, IL-1, and tumor necrosis factor-a (TNF-α). The main anti-inflammatory cytokine includes IL-10. The levels of interferon Gamma (IFN-γ) and TNF are changed by general anesthesia (10). Evaluation of the effects of general anesthesia agents on the immune system helps to improve the management of anesthesia. This review focuses on the effects of the general anesthesia on the immune response.
2. Evidence Acquisition
The current review aimed to summarize the literature about the effects of general anesthesia on the cytokines. Using several keywords, including cytokine, general anesthesia, immune response, intravenous anesthetics, volatile anesthetics, inherent immune system, acquired immune system, opioids, benzodiazepines, and controlled ventilation the following databases were searched: Google Scholar, PubMed, and ISI/Web of Sciences. To complete the search, references of the identified studies were also reviewed.
The authors independently search the literature, then all relevant articles were reviewed. To facilitate the review, EndNote X8 was used. To determine the eligibility of identified studies, the titles and abstracts of the studies were reviewed. Further assessment was done through full-text reading concerning the inclusion criteria. The following data were extracted from the included studies: The type of drug used in general anesthesia, the type of cytokine, and the effects of the drugs on cytokine.
2.1. Inclusion and Exclusion Criteria
Articles on the effects of the drugs used to produce general anesthesia on the immune system were included. Studies about the regional or local anesthesia effects on the immune system were excluded. Papers not written in English and conference abstracts were also excluded.
3. Results
3.1. Effects of Intravenous Anesthetics on Cytokines
The most common method to produce general anesthesia is the intravenous anesthesia (IV anesthesia). Sodium thiopental, propofol, and ketamine are examples of drugs which commonly use in IV anesthesia (14). Ketamine and sodium thiopental reduce the number of T-helper cells and NK cell activities and increase the T-inhibiting cells (15).
Sodium thiopental and ketamine inhibit the release of IL-1, IL-6, TNF-α, and IL-8. It is well-documented that low dose ketamine, as an N methyl-D-aspartate (NMDA) receptors antagonist, reduces the lifetime of IL-6 (16, 17). In addition, these drugs increase the level of IL-10 (18). However, according to the results reported by Song et al. (19), Ketamine inhibits the production of anti-inflammatory cytokines.
Sodium thiopental is a rapid induction intravenous anesthetic agent which affects the GABA- A receptors that their presence in immune cells is confirmed. In a study, the effect of propofol and sodium thiopental on Th1/Th2 balance is compared by measuring the levels of IFN-γ, IL-4, and IL-2. Compared to propofol, sodium thiopental reduced the concentration of IFN-γ and IL-4 without affecting the concentration of IL-2 (20).
Propofol (2,6-diisopropylphenol) is a commonly used intravenous anesthetic agent. Besides, it uses for maintenance of general anesthesia and sedation in the intensive care unit. Propofol causes rapid induction of and recovery from anesthesia. This drug has anti-oxidant and anti-inflammatory characteristics (21). Information about whether anesthesia with propofol increases or decreases the IL-6 levels is controversial. Propofol in vitro inhibits IL-6 production by stimulated lipoproteins (22). The study conducted by Gonzalez-Correa et al. (23) reported that propofol decreased the concentration of IL-1, TNF-α, and IL-6 cytokines. Propofol increases the concentration of IL-10. A higher concentration of anti-inflammatory cytokines in patients who received propofol is the reason for its anti-inflammatory properties (24, 25). Jin et al. (20) reported that Injection of high dose propofol increased the ratio of IFN-γ/IL-4, while low dose propofol did not change the concentration of the cytokine.
Fekkes et al. (26) reported that IL-6 concentration increases during the surgery. They showed that the duration of surgery was the main determinant of IL-6 response and propofol does not seem to affect the response of IL-6. Zhao and Mo (27) found that in general anesthesia, the content of T-lymphocyte subsets and NK cells descended significantly (P < 0.05).
3.2. Effects of Inhalational (Volatile) Anesthetics on Cytokines
Volatile anesthetics often uses to induce and maintain general anesthesia. The volatile nature of these compounds extends their influences to the immune system of the patients, physicians, nurses, and other personnel in operation and post-operation rooms (28). The inhibitory effect of volatile anesthetics on the immune function is confirmed (29). Volatile anesthetics can inhibit the release of IFN in animals, reduce the number of NK cells, and the production of cytokines in the human body (30).
Halothane and enflurane do not administer in clinical settings, therefore we will not discuss them in this review. Recent studies showed that the clinical concentration of isoflurane, which commonly uses in general anesthesia, can alter beta-adrenergic responses in rats (31). It has been shown that isoflurane increases the level of IL-6. Tylman et al. (32) investigated the effects of sevoflurane on inflammatory responses of the immune system and reported an increase in IL-8 and a decrease in IL-17 concentrations. Cho et al. (33) reported that sevoflurane reduces the cytokine release, particularly IL-6, IL-8, and IL-10. Results of the study conducted by Sofra et al. (34) are consistent with the study conducted by Kvarnsrtom et al. (35) that reported an increase in IL-6 levels in patients who had abdominal surgery by using sevoflurane. Some studies reported that the sevoflurane suppresses the production of IL-6, TNF-α, and IL-10 (36-38).
Desflurane increases the number of neutrophil cell and pro-inflammatory cytokines expression in alveolar macrophages (38). Desflurane produces more pro-inflammatory responses compared to sevoflurane (39). Kalayci et al. (7) evaluated the plasma level of IL-10 in response to desflurane with two different flow rates. The results showed that the plasma concentration of IL-10 at the end of surgery and 24-hours later was significantly higher compared to the start of surgery.
3.3. Effects of the Opioid on Cytokines
During general anesthesia, opioids administer to produce analgesia and deepen its levels. Evidence suggests that opioids affect the immune system, although the results are contradictory (40). Both the suppressive and stimulant effects of the opioids on the immune system are reported by various studies. Opioids cause a significant reduction in the concentration of TNF-α, IL-1, and IL-6 (41). The study conducted by Makimura et al. (42) reported a correlation between the concentration of some cytokines (IL-8, IL-12, MIP-1α) and pain tolerance after administration of morphine. Morphine produces TGF-β by stimulating lymphocytes, monocytes, and macrophages. These effects are mainly the result of long-term treatment, which leads to an increase in the concentration of IL-4 and IL-5 as well as the reduction of IL-2 and IFN-γ (33). Vassou et al. (43) provided evidence that opioids can regulate humoral immunity by decreasing the antibody secretion of B-lymphocytes.
Fentanyl exhibited cytotoxicity against NK cells. Forget et al. (44) reported that fentanyl inhibits the activities of NK cells in mice. Beilin et al. (45) found that 24 hours after the operation, the activity of NK cells was reduced with both low and high doses of fentanyl. Some studies reported that opioids can enhance immunity by enhancing the activities of the NK cell (46, 47).
Tramadol is a class of weak opioid receptor agonists that plays an important role in the current clinical “multimodal analgesia” concept. These drugs exhibited protective effects on cellular immunity in some studies.
It is found that morphine, remifentanil, fentanyl, methadone, and codeine alter the immune system more than oxycodone, hydrocodone, and tramadol (48). Remifentanil decreases IFN-γ concentrations. IL-6, TNF, IL-10, and IL-2 concentrations do not change during treatments with remifentanil or fentanyl.
3.4. Effect of Benzodiazepines on Cytokines
Benzodiazepines are one of the most commonly used sedatives in general anesthesia and intensive care units (14). Hypnotic drugs influence the cytokines in vitro, but the results are not definitive (22). Midazolam is more common than other benzodiazepines, because if administered intravenously causes less pain, and it’s a short-acting drug compared to others (49). Midazolam can inhibit the production of IL-2 and IL-8 and produces an immunosuppression effect (50). Midazolam can inhibit the activity of TNF-α (51). Rapid administration of diazepam will produce a pro-inflammatory response, but its continuous administration (60 days or longer) will inhibit the leukocyte function and lead to immune response inhibition (52). These results show that benzodiazepines have a significant inhibitory effect on immunity, but more research should be conducted on adaptive immunity.
3.5. Effect of Controlled Ventilation During Anesthesia on Cytokines
One of the ventilation methods during general anesthesia is controlled ventilation. Controlled ventilation, through damaging alveolar cells and mechanical stress, suppresses the stability of the ventilation/perfusion ratio, which results in the release of inflammatory mediators and worsening of the gas exchange during the postoperative period (53). Lung tissue damage directly influences the airway volume, lung capillary trauma, and neutrophil accumulation (54).
The other factor that causes lung tissue damage and increases the release of cytokines is hypoxia during surgeries. Schilling et al. showed that the type of ventilation and general anesthesia agent affect the concentration of pro-inflammatory cytokines (27). Sevoflurane reduces the inflammatory response during one-lung ventilation in chest surgery and may be preferable in patients who the level of their pro-inflammatory cytokines is more than expected (54). The effects of general anesthesia on innate and acquired immune system cytokines are summarized in Figure 2.
4. Discussion
Intravenous anesthetics have anti-inflammatory properties, which in most septic cases are useful for patients. The anti-inflammatory effects of ketamine may be related to the suppression of TNF production by macrophage in the presence of bacteria (55). It seems that ketamine and sodium thiopental have detrimental effects on the mast cells in patients with a high risk of infection.
According to the results, propofol inhibits the phagocytosis and chemotaxis of human monocytes through GABAA receptors (26).
Inhalational anesthetics have several effects on initiate immunity, particularly through influencing neutrophils, DCs, NKs, and macrophages (50, 56). Inhalational anesthetics, in a dose-dependent manner, suppress cytokine release, reduces lymphocyte proliferation, induce apoptosis of the lymphocytes, and inhibits the function of neutrophils (57). Besides, to the direct effects, inhalational anesthetics influence the endocrine response from the hypothalamus-pituitary-adrenal axis and indirectly through the secretions of hormones, such as glucocorticoids and catecholamines (58).
There are also evidence about the association between adrenergic receptor activities and cytokine production. In stressful situations, elevated concentrations of epinephrine can trigger IL-6 secretion through β2 adrenergic receptors. While the activation of α2 adrenergic receptor in the macrophage membrane can increase TNF-α secretion (59). Studies that investigated the effects of inhalational anesthetics on cytokines production have reported different results (16, 60). In animals, IL-6 elevation is associated with impaired learning and memory (61, 62). It is conceivable that isoflurane, by elevating the IL-6 concentration in the brain, reduces neuronal behaviors in animals, which may be related to the decreased cognitive function of isoflurane in rodents (63, 64) and (possibly) in humans (65). However, the mechanism through which the isoflurane increases IL-6 should be identified (66, 67). The study conducted by Lin and Zuo (68) showed that isoflurane activates the IL-1 pathway and causes cellular damage to the hippocampus, which in animal models may lead to cognitive impairment. Miyata et al. (69) reported a significant decrease in NK cytotoxic activities, 24 hours after isoflurane anesthesia.
Schneemilch et al. (16) investigated the effects of anesthetic agents, on proliferation and the production of cytokines. Sevoflurane, in combination with sodium thiopental or nitrous oxide, compensate inhibitory effects of sodium thiopental and nitrous oxide. Fentanyl, sufentanil, sevoflurane, and nitrous oxide did not affect the level of IL-2 and release of SIL-2R. They conclude that sodium thiopental and nitrous oxide have immunosuppressive activities and, on the contrary, sevoflurane may have beneficial effects by reducing the sodium thiopental inhibition (16).
Opioids exert its effects either directly through the receptor (µ, δ, and κ), which are widely present in neurons and immune cells, or through the autonomic and the central nervous system. (70-72). In fact, it is shown that opioids that cross the blood-brain barrier have more moderating effects on the immune system. Opioids affect the innate and acquired immune system, including the synthesis of cytokines and immunoglobulins as well as activation of NK and phagocytosis (70, 73). Opioid receptors typically express by immune and peripheral glial cells.
Morphine activates glial cells which in turn results in the release of cytokines, including IL-1β, IL-6, and TNF-α, countered with the analgesic effects of morphine. The release of cytokines is not associate with the frequency and duration of morphine administration. Given the short-term response of cytokine to morphine (about 5 minutes), it can be assumed that morphine stimulates the release of stored cytokines, rather than synthesizes them. The study of Shavit et al. (74) reported that IL-1 could reduce morphine-induced analgesia. The authors also noted that IL-1 plays an important role in tolerance to morphine. On the other hand, Byrne et al. (75) found that IL-1β affects the expression of opioid receptors in glial cells, and IL-1β can regulate opioid receptors (µ, δ, and K) in astrocytes. Synthetic opioids produce more transient changes in the immune system (76). The inhibitory effects of sufentanil and alfentanil on leukocyte migration and the activity of natural killer cells are reported in various studies (77). Tramadol increases the postoperative NK activities in patients with cancer (78, 79).
Midazolam exerts its effect through central benzodiazepine receptors (CBRs) and peripheral benzodiazepine receptors (PBRs) (80). Expression of PBR on the macrophage surface allows benzodiazepines to regulate the pro-inflammatory function of macrophages by blocking their ability to produce superoxide anions and IL-1, TNF-α, and IL-6 cytokines. Midazolam decreased the IL-8 release in lipopolysaccharide-induced neutrophils (81). The suppression of IL-8 release may increase the risk of infection in the postoperative period. Activation of leukocytes through LPS in the presence of midazolam reduces the extracellular concentration of IL-8, while the intracellular concentration remains unchanged (20).
5. Conclusions
Several studies investigated the anesthesia effects on the immune system, although some aspects remain unknown yet. For this reason, anesthesiologists should be aware of the benefits and disadvantages of different anesthesia agents as well as techniques to induce anesthesia. Particularly anesthesiologists should choose the preferred anesthesia method for patients with an imbalance in their immune systems to prevent further loss. It seems that the effects of general anesthesia on the immune system are not considerable in healthy patients and short-term surgeries and changes in the immune system on the first day after surgery are mainly due to surgical trauma. Long-term administration of general anesthesia drugs, due to their effects on cytokines, can lead to disease progression in patients with immune deficiency. Because the results of various studies are conflicting and since the number of patients with an immune deficiency is rising, choosing the appropriate general anesthesia agents facilitates the more favorable function of the cytokines.
Research on human specimens affect various variables such as gender, duration of anesthesia, operating room temperature, anesthetic drugs, surgical procedure, and surgical stimulus. In the current study, such variables make it difficult to determine whether immunosuppression perioperative is a side effect or an advantage. Controlled randomized clinical trials are desirable to find the effect of a short period of immunosuppression on patient outcomes. Because immunosuppression is detrimental for cancer patients, but beneficial for septic patients, choosing the appropriate anesthesia method and agents should be carefully evaluated.