1. Background
Trigeminal autonomic cephalalgias (TACs) are a group of headaches characterized by unilateral head and/or facial pain in association with autonomic signs and typically occur with a circadian and annual rhythm. The prevalence of these cephalalgias is less than migraine, but they are very debilitating and significantly affect the patients' quality of life (1).
Cluster headache (CH), the most common type of TACs, is a variant of primary neurovascular headaches and is characterized by a unilateral, recurrent, and short-lived pain episode that has symptoms of autonomic system dysfunction. With no treatment, pain episodes last for 15 to 180 min. CHs are classified into two groups of chronic and episodic headaches. Approximately 80% of all cases are episodic and occur once or twice a year. Among 20% of the patients, CH is a chronic disease. These patients experience recurrent headache attacks for one year or more, with remission periods of less than a month (1, 2).
Medical treatments are focused on the treatment of the current acute attack and the prophylaxis of future headaches. Some patients do not adequately respond to medical treatments and might benefit from interventional management. As inadequate pain management is very common and can prolong pain duration (3, 4), efforts, such as interventional pain management should be tried to manage intractable pain.
The sphenopalatine ganglion (SPG) is the largest extracranial neural structure located in the pterygopalatine fossa. This ganglion consists of sensory, motor, and autonomic (mostly parasympathetic) neurons and plays a role in the pathophysiology of the CHs (5-7).
SPG block and radiofrequency (RF) denervation have been already proposed for the treatment of CH and were the standard treatments for this headache in the pain literature; however, few studies have evaluated the extent and duration of the efficacy of this intervention (5, 7, 8).
2. Objectives
The purpose of this investigation was to evaluate the efficacy, the duration of pain relief, and complication(s) of the SPG block and conventional RF denervation in the treatment of CH.
3. Methods
The ethics committee of the university approved the research protocol (Code: IR.TUMS.IKHC.REC.1397.030) that was in accordance with the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a prior approval by the institution's human research committee. We did not assign our patients to any new intervention. Our patients were assigned to this intervention regardless of our study based on the fact that this intervention had already been recommended for the treatment of CH in the literature. This was a follow-up study, and we provided the data of a long-term follow-up of our patients in this report.
Patients with CH who received medical therapy, including acetaminophen, nonsteroidal anti-inflammatory drugs (NSAIDs), and oxygen, for at least six months and were resistant or intolerant to this treatment were referred to our pain clinic by the neurology service of our hospital between 2014 and 2018. We extensively evaluated the patients and reviewed their past medical, social, and dug histories.
We obtained informed consent from all patients. Patients with the following criteria were candidates for the SPG block/denervation in our pain clinic 1) age between 17 and 75 years old; 2) all patients with CH who met the International Classification of Headache Disorders (ICHD-3) criteria and were referred to our pain clinic; 3) patients who were intolerant or had an inadequate response to medical therapy for at least three months; and 4) patients who were willing to receive interventional management. Exclusion criteria were: 1) any coagulopathy; 2) any infection/lesion on the path of needle insertion; 3) pregnancy.
We registered the following information for all patients: age, sex, and pain intensity (PI). We used a validated version of the Brief Pain Inventory (BPI) to follow the patients and collect data (9). The BPI is a multidimensional questionnaire and has four numeric rating scales (NRS) to measure pain intensity at its least, worst, average, and current severity. BPI also has some questions about pain relief and interference with function, enjoyment, and mood. Patients can show their pain intensity by an 11-point NRS with four questions: minimal, maximal, right-now (at the time of the interview), and overall (average pain intensity that the patient had during the last week). The ‘mean PI’ was defined as the mean of the maximal and overall pain intensities. Eventually, the ‘mean PI’ was calculated and recorded for each patient.
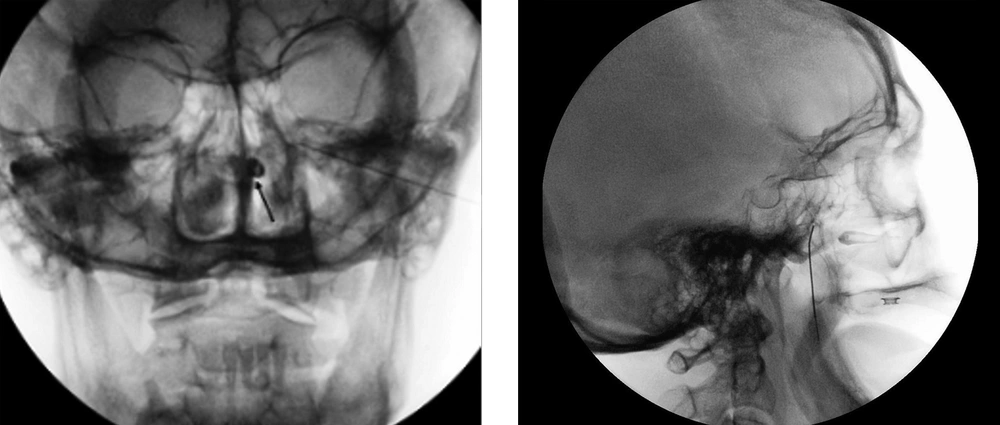
The treatment plan had two parts: prognostic and subsequent SPG therapeutic block (RF denervation). During the prognostic block, C-arm-guided infra-zygomatic approach was chosen to locate the sphenopalatine fossa (Figure 1). Patients were placed in a supine position. We performed the block under ASA standard monitoring (electrocardiogram (ECG), SpO2, heart rate (HR), respiration rate (RR), non-invasive blood pressure (NIBP)), and used a 22G spinal needle. We sedated the patients with 1 mg IV midazolam and 50 µg fentanyl. We employed 10 ml of 0.5% lidocaine as the skin local anesthesia. The needle was placed over the supramandibular notch and was directed toward the sphenopalatine (SP) fossa. Once the needle tip was in the correct position (approved by the fluoroscopic views), we performed the prognostic SPG block using a 2 ml solution, including 4 mg dexamethasone and 1% lidocaine. We visited every patient two days after the prognostic block and evaluated the clinical response by filling the BPI. If the patients had at least 50% pain reduction within the first 5 h after the injection, we scheduled them for the therapeutic block (RF denervation), usually one week later. A Radiofrequency needle (100 mm, active tip = 5mm, 22G, sharp, curved) was used (R-FTM Needles-Epimed). Sensory stimulation was initiated at 50 Hz and the stimulation intensity was gradually increased to 0.5 V (RF device: Neurotherm NT2000iX). The satisfactory response was the sensation of tingling in the nasal root. Consequently, the tip of the needle was in the right place, adjacent to the SPG, and far from maxillary or palatine nerves. After getting the acceptable sensory stimulation, we injected 1 ml of 1% lidocaine before the ablation, and subsequently, we performed the neurolysis using the conventional RF denervation (temperature: 80°C, duration: 90 sec 2 cycles/point, and at two close points). We followed the patients by filling the BPI questionnaire at 48 h, and 1, 3, and 6 months after the denervation.
3.1. Statistics
Collected data are presented as mean, range, standard deviation, and minimum and maximum effect in SPSS version 25 (IBM, New York, NY, USA).
4. Results
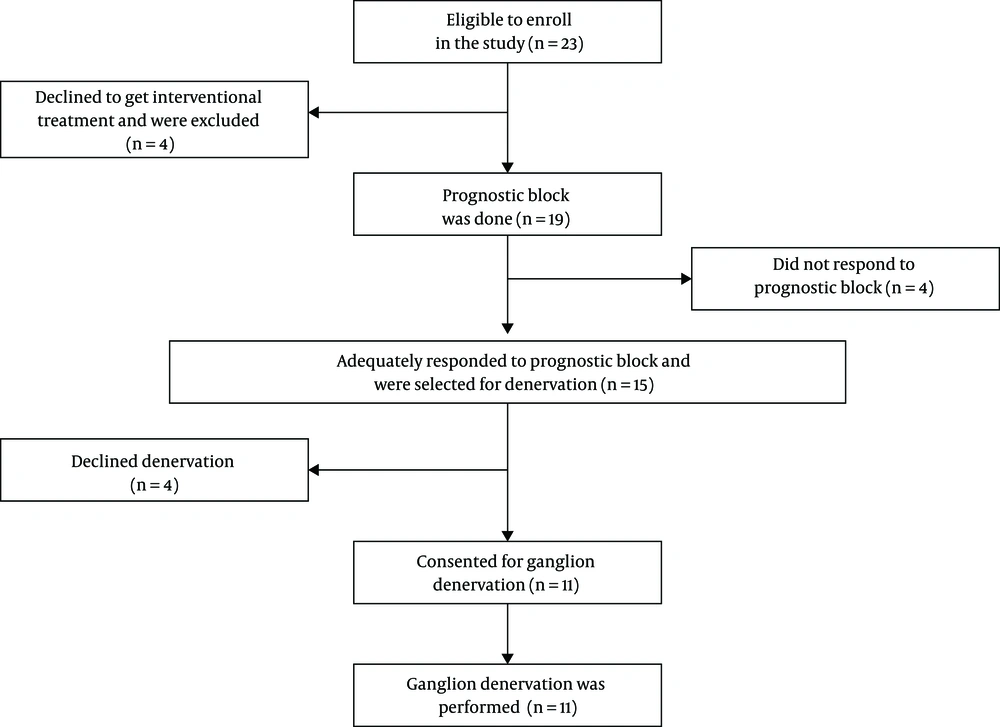
Among the patients with CH who were referred to our pain clinic, 23 subjects met our criteria. All 23 subjects consented that we recruit their data in our study; however, four patients refused to undergo the interventional treatment. Nineteen patients consented to the SPG prognostic block (Figure. 2). Patients’ characteristics are demonstrated in Table 1.
| Variable | Values |
|---|---|
| Age (y), mean (range) | 46 (17 - 75) |
| Gender | |
| Male | 12 (53) |
| Female | 11 (47) |
| Pain intensity, mean ± SD | |
| Minimum | 1.45 ± 1.43 |
| Maximum | 9.36 ± 1.06 |
| Overall (average) | 6.73 ± 1.48 |
| Mean PI, mean ± SDb | 8.04 ± 1.27 |
aValues are numbers (percentages) unless stated otherwise.
bPain intensity was evaluated by NRS; mean PI, mean Pain Intensity for each patient, the mean among the highest, and overall pain intensities for each patient.
Fifteen out of 19 patients (79%) had an acceptable response to prognostic block, which was more than 50% pain reduction. Also, 11 out of 19 patients (57%) reported a 100% pain reduction after the procedure. Four out of 19 patients (21%) did not express adequate relief, and these four patients were excluded from the study. Mean pain reduction after the prognostic block was 75% (Table 2). Among those who were selected for SPG denervation (n = 15), 11 patients consented to undergo the ablation. Financial issues, fear of adverse effects, and short-lasting outcomes were mentioned as the reasons for refusal. We followed the patients as long as they had not used other treatments. Initial pain intensity and pain reduction 48 h, and 1, 3, and 6 months after SPG denervation are demonstrated in Table 2.
aMean Pain Intensity: the mean among the highest and overall pain intensities for each patient.
bThe values are mean ± SD.
cAfter SPG Denervation.
We performed 30 procedures (19 prognostic blocks and 11 denervations) in this study. There were only two complications after the denervation in our patients. One patient demonstrated soft tissue infection, which was successfully treated by antibiotics. The second patient had a sensory deficit over the maxillary dermatome of the trigeminal nerve, which lasted for one year.
5. Discussion
Some patients with cluster-type cephalgia develop a chronic and drug-resistant headache that is not responsive to conventional treatments. Invasive procedures, including SPG block/denervation, are proposed to relieve their intractable pain (5, 10, 11). We found that pain intensity decreased by 50% or more at 48 h, and 1 and 3 months after denervation. At 6 months’ follow-up, pain intensity decreased by 31%.
There are very few controlled studies on the efficacy of the SPG block/RF denervation in treating the CH in the literature (5, 8). Systematic reviews demonstrated the short-term efficacy of SPG block in the treatment of CH, which was compatible with our study. However, many studies have been conducted on the transnasal approach, whereas our report was on the infrazygomatic technique. They concluded that there is moderate evidence (grade of recommendation B) for SPG block in treating CH.
The available systematic reviews separately evaluated the efficacy of the SPG denervation in addition to the evaluation of the simple blocks. They showed that the grade of recommendation was also B for RF denervation. Our study was in line with these reports; however, the duration of pain reduction was shorter in our study (5, 8).
Narouze et al. showed that percutaneous SPG RF denervation is a highly effective treatment for chronic CH. While the pain disability index (PDI) was 55 at the beginning of the study, it diminished to 17.2 and 25.6 at 6 - and 12-months post-procedure, respectively. Also, the mean attack intensity at 1, 3, 6, 12, and 18 months after the procedure were 2.6, 3.2, 3.2, 3.4, and 4.2, respectively. They concluded that SPG denervation is an effective method in the treatment of chronic CH (6). Our findings were also similar to their results and demonstrated that the patients with CH could benefit from the SPG RF denervation at least for several months. However, the efficacy of the procedure was reduced in our patients after some months.
Sanders et al. found similar results in their study. They evaluated the efficacy of the SPG RF denervation in CH treatment during a follow-up period of 12 to 70 months. Sixty-six drug-resistant CH patients were enrolled in this study, of whom 56 patients had experienced episodic attacks and 10 had suffered from chronic headaches. All of the patients underwent radiofrequency ablation. Complete pain relief was seen in 34 patients (60.37%) of the episodic group and three patients (30%) of the chronic group. Eight patients (14.3%) of the episodic group and four patients (40%) of the chronic group stated no pain relief (12). We could show comparable results in our study, as well. Our patients had considerable pain relief. However, the follow-up period was shorter and over time, the efficacy of the treatment diminished in our report.
Side effects of this procedure are mostly temporary and include infections, epistaxis, hematoma formation, anesthesia, or hypoesthesia in the palate and pharynx (5). Using real-time fluoroscopy has decreased the incidence of these complications (5, 7). In this study, a total of 30 interventions, including prognostic block and neurolysis were done, of which only two had side effects. The side effects were soft tissue infection and sensory deficit over V2 (maxillary nerve dermatome). They were managed successfully.
This report has some limitations. We did not report the change of the patients’ analgesics after our interventions. Our patients could not be the representatives of the general population.
5.1. Conclusion
SPG conventional radiofrequency denervation can effectively decrease the pain intensity of the CH with acceptable side effects for at least several months.