1. Background
Spinal anesthesia is a safe method for delivery via cesarean sections because it can be used more easily and faster than epidural anesthesia. It can also lead the fetus to have less exposure to drugs than in general anesthesia, and the pregnant patient can wake up at the birth of her baby. The most common side effect of this procedure is the reduced maternal blood pressure, which can cause 90% of mothers to experience complications such as nausea, vomiting, dizziness, fetal acidosis, and even cardiovascular collapse and fetal bradycardia in extreme cases (1). Hypotension due to decreased vascular resistance in pregnant women is exacerbated by compression on the inferior vena cava, which is relatively compensated through the heart rate and augmented stroke volume (2). The foremost prevention method of hypotension in the caesarian section is questionable. A novel agreement training recommends the prophylactic administration of vasopressors used for the cesarean section. There are several ways to reduce this complication, such as using the proper maternal position with uterus displaced off the vena cava, infusion of fluids, physical interventions such as leg wrapping, using compression mechanical pump device (e.g., Sequential Compression Device (SCD)), and prophylactic vasopressors (3). In the method of using a compression mechanical pump device, the pressure recurrently is exerted upper the knee. Based on past studies, blood with an amount of nearly 125 cc is motivated in the compression phase. During pregnancy, a high volume of blood exists in lower extremities that is additionally elevated by spinal anesthesia via vasodilatation (4). Therefore, SCD reduces hypotension by increasing the central volume in a highly robust manner, while other approaches have, to some extent short-term and transitional effect (5).
2. Objectives
This study evaluated the efficacy of SCD in preventing hemodynamic changes following spinal anesthesia for the cesarean section.
3. Methods
3.1. Trial Design
This study was a prospective double-blinded randomized clinical trial with a parallel assignment of patients at a ratio of 1:1 in two groups conducted after approval by the Ethics Committee of Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran (code: IR.AJUMS.REC. PAIN-9312). The study carried out from December 2017 to January 2018 in Razi Hospital, Ahwaz, Iran. The RCT code of this study was IRCT2015011217742N3. Written informed consent was collected from all of the patients. During the study period, of 100 pregnant patients undergoing elective cesarean sections, 73 patients were selected to contribute to the study. They were divided into the SCD group (35 patients) or the control group (38 patients) (CONSORT flow diagram).
3.2. Participants
The inclusion criteria included non-laboring parturient women, American Society of Anesthesiologists (ASA) I or II, age of 18 to 45 years, and elective cesarean sections.
The exclusion criteria were the contraindication of spinal anesthesia, multiple gestation, complicated pregnancy, heart diseases, peripheral vascular disease, hemodynamic instability, known sensitivity to local anesthetic, Body Mass Index (BMI) > 40, and gestational diabetes.
3.3. Randomization
Simple random sampling was used to classify the patients into SCD (S) and control (C) groups at a 1:1 ratio. One of the researchers with no role in the treatment of patients carried out randomization.
3.4. Study Setting and Interventions
All patients were evaluated before surgery by an anesthesiologist that was blind to the research. Relevant laboratory investigations (complete blood count, coagulation profile, liver function, and renal function tests) were done. In the operating room (OR), simple random sampling was used to divide the patients into SCD and control groups. In the OR, all the patients were placed in a position with a lateral angle of 15 degrees toward left. The patients were monitored continuously for electrocardiogram changes and pulse oximetry. Accordingly, the baseline systolic arterial pressure (SAP), diastolic arterial pressure (DBP), and mean arterial pressure (MAP) along with heart rate (HR) were documented by a non-invasive oscillometric blood pressure monitor. Volume loading was administrated with Ringer’s lactate solution 10 mL/kg for 10 min, and then it continued at a rate of 10 mL/kg/h as the maintenance fluid. Sleeves with proper sizes were applied to lower limbs of the patients in both groups. Patients in the SCD group received discontinuous compression at 50 mmHg pressure. Using a 25 G Sprotte needle, an expert anesthesiologist blinded to the study performed spinal anesthesia in the L3-4 or L4-5 interspace in a sitting position. Intrathecal injection to the patients yielded 10 mg of bupivacaine 0.5% with 2.5 µg sufentanil.
Consequently, the patients were placed in a supine position with a lateral angle of 15 degrees toward left. Five minutes after SA, the upper sensory level was detected in anesthesia. Moreover, during each minute of the experiment, the arterial blood pressure was measured until delivery. After that, the procedure was repeated and recorded in the following minutes: 3, 5, 10, 15, 25, 35, 45, 60, and 75 min. Hypotension was described as a reduction below 20% of the baseline in MAP or below 90 mmHg in SAP. Bradycardia was described as a reduction of HR below 60/min. An anesthesiologist blinded to the protocol and study groups treated potential hypotension and bradycardia using 5 mg intravenous ephedrine increment boluses simultaneously with the administration of the intravenous fluid.
The primary outcomes were blood pressure and heart rate changes. The secondary outcomes were vomiting or nausea along with Apgar scores at 1 and 5 minutes for the neonate. In the case of nausea and vomiting, metoclopramide was prescribed. If hypotension occurred, ephedrine was prescribed, and the total dose of ephedrine was recorded.
3.5. Sample Size
The study population consisted of 60 patients (30 in each group) according to a confidence interval of 95% and power of 90% for hemodynamic changes after spinal anesthesia based on a previous study (3). Nevertheless, we increased the sample size to 50 patients in each study group to account for any withdrawal or missing data points.
3.6. Statistical Analysis
GraphPad Prism 6 (graph [ad®., Chicago, Illinois, USA) was used to perform statistical analyses. The data were reported as mean (± SD), median (range), or numbers (%). The P values of below 0.05 were regarded as significant. The student’s t-test was used to compare the groups in terms of normally-distributed continuous variables. Also, the chi-square test was used to compare the groups in terms of nominal categorical data. Moreover, repeated-measures RMANOVA was used to analyze serial data and demonstrated three significance levels. Finally, the chi-square test was used to compare the groups in terms of hypotension incidence rate.
4. Results
During the study period, 73 pregnant patients completed the study. There were no significant differences between the groups in the patients’ characteristics, maximum sensory block, skin incision to delivery time, spinal anesthesia to delivery time (min), and the total duration of surgery (Table 1).
| Variable | SCD Group (N = 35) | Control Group (N = 38) | P Value |
|---|---|---|---|
| Age, y | 30.2 ± 1.65 | 29.5 ± 2.1 | 0.65 |
| Height, cm | 155 ± 4.8 | 154 ± 5.1 | 0.13 |
| Weight, kg | 65 ± 7.8 | 69 ± 8.5 | 0.10 |
| Gestational age | 38.3 ± 0.85 | 38.4 ± 0.75 | 0.22 |
| Maximum sensory block (T) | T5 (T4-T6) | T4 (T4-T6) | 0.94 |
| Skin incision to delivery time, min | 7.85 ± 6.5-12 | 8.24 ± 6.8-10.2 | 0.75 |
| Spinal anesthesia to delivery time, min | 18.25 ± 3.2 | 18.45 ± 2.4 | 0.67 |
| Duration of surgery, min | 40.35 ± 4.15 | 39.45 ± 3.20 | 0.65 |
aValues are expressed as mean ± SD.
bThe number of patients, or median at P < 0.05 as statistically significant.
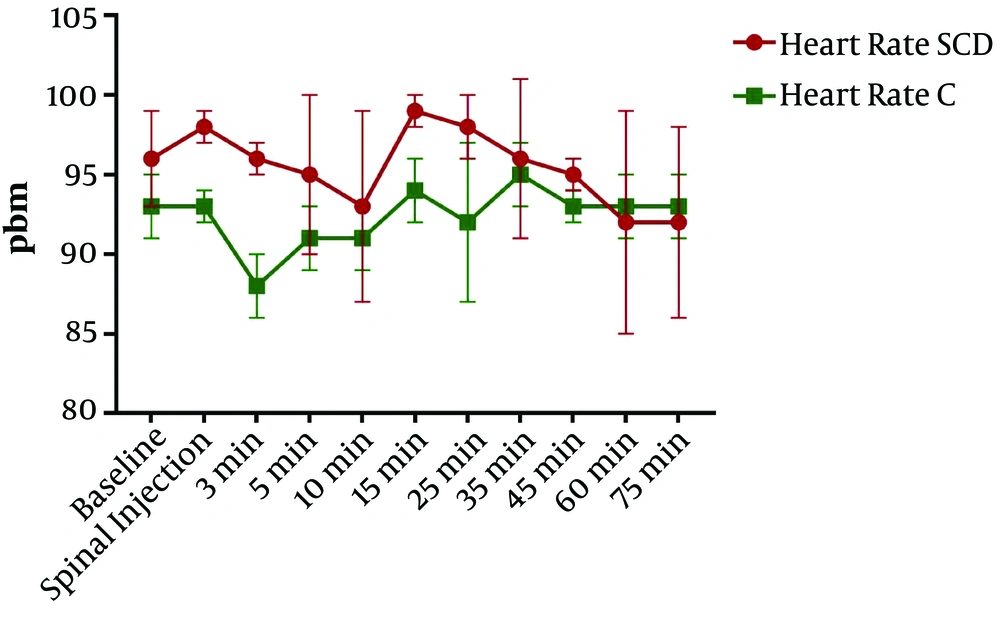
Concerning heart rate changes, RM ANOVA showed the significance of the effect of time, group, and interaction of the two factors (P < 0.0001, P < 0.0001, and P < 0.0001, respectively). Repeated-measures ANOVA is a type of two-way ANOVA that indicates three P values, including the first one for significant changes in the time, the second one for significant changes in each group, and the third one for the interaction of both. Tukey post hoc test showed that the heart rate was significantly greater three and 15 min after spinal anesthesia in the SCD group than in the control group (P < 0.05) (Figure 1).
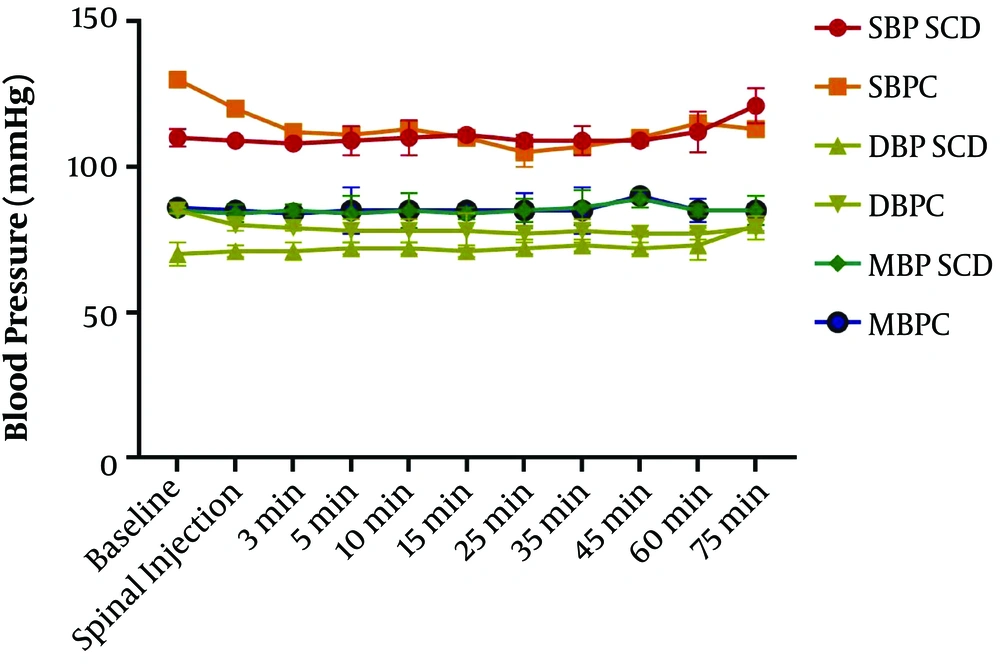
Comparison of changes in blood pressure between the two groups showed mean blood pressure (85.18 ± 1.4 in SCD vs. 85.45 ± 1.5 in C), systolic blood pressure (110.6 ± 3.61 in SCD vs. 113.3 ± 6.81 in C), and diastolic blood pressure (72.45 ± 2.66 in SCD vs. 78.73 ± 2.28 in C). RM ANOVA showed a difference in the effect of time, groups, and the interaction of the two factors (P < 0.0001, P = 0.35, P = 0.77, respectively). Repeated-measures ANOVA is a type of two way ANOVA that indicates three P values, including the first one for significant changes in the time, the second one for significant changes in each group, and the third one for the interaction of both.
Tukey post hoc test showed three minutes after spinal anesthesia, diastolic blood pressure was significantly higher in the SCD group than in the control group (P < 0.05). The differences between the two groups at other times in systolic, diastolic, and mean blood pressure were not significant (Figure 2).
The SCD group showed to have significantly lower rates of nausea (eight subjects in the SCD group (24%) against 18 subjects in the C group (48%), p=0.005) and vomiting (n = 4 in the SCD group (11%) against n = 8 in the C group (21%), P = 0.001). Further, the SCD group showed to have a significantly lower mean ephedrine dosage per patient (4.1 mg against 17.1 mg, P = 0.001).
However, no meaningful difference was detected between the groups regarding the neonatal Apgar scores at one (P = 0.65) and five (P = 0.9) minutes.
5. Discussion
Spinal anesthesia-induced hypotension results from decreased tonicity in arteriolar and venous circulation secondary to the sympathetic block, thus inducing a decrease in systemic vascular resistance and spreading the central blood volume to the peripheral compartments (6). There are several different ways to prevent or decrease hypotension following spinal anesthesia. Nonetheless, there is no recognized ideal method. The most common involvements that have been used are prophylactic fluid hydration (crystalloid or colloid), usage of vasopressors like ephedrine or phenylephrine, and usage of varying mechanical interventions for increasing central blood volume such as leg bandages.
Preloading with crystalloids has been questionable in the prevention of hypotension, while colloids are related to the high charge and opportunity of hypersensitivity and weakened coagulation. The use of vasopressors damages the perfusion of the Uteroplacenta due to vasoconstriction, thus leading to fetal or neonatal side effects. Since venous blood pooling in the leg and abdomen is effective in hypotension due to spinal anesthesia, we considered SCD as a way to decrease the incidence and severity of hypotension. In this study, we found no significant difference in hypotension between the groups. However, the administrated ephedrine dosage was meaningfully lower in the SCD group than in the control group.
Moreover, the SCD group had meaningfully lower vomiting and nausea incidence rates than the control group. The incidence of nausea and vomiting with no prior prophylaxis occurs in up to 80% of all patients after spinal anesthesia for cesarean sections (7). It can be caused by the induced temporary sympathectomy, changes in blood pressure in terms of significant hypotension, and bradycardia due to increased vagal tone (8). In our study, heart rates were higher in the SCD group at 3 and 15 minutes. Although sympathetic block produces hypotension and bradycardia, reflex tachycardia occurs to compensate for heart physiology. Also, the volume of venous return increases from lower limbs in the SCD group simultaneously that cause tachycardia. 15 minutes after spinal anesthesia is the time after the delivery. Therefore, a large volume of blood enters the heart, which can cause tachycardia to reduce the volume overload. This phenomenon is prominent in the SCD group due to the increased venous return from the lower limbs.
Some interventions, such as leg elevation, leg wrapping, and application of leg compression through elastic stocking, can reduce bradycardia, nausea, and vomiting by increasing the central volume (9, 10). In a study by Sujata, 100 pregnant women receiving elective cesarean sections under spinal anesthesia were divided into two groups of pneumatic compression and control. Both groups received similar spinal anesthesia and standard pre-surgery fluid therapy protocols. Hypotension was treated with ephedrine. The treatment and control groups had 25.5% and 60% hypotension rates, respectively (P = 0.001). In our study, the incidence of systolic blood pressure was 51% ± 21 in the control group and 45% ± 20 in the SCD group, which did not differ significantly (P = 0.28). The result of the study by Sujata et al. (11) about the incidence of hypotension is not in line with our study findings. However, in their study, the median dosage of ephedrine was 12 [0 - 24] mg and 0 [0 - 12] mg in the control and treatment groups, respectively (P < 0.001), which is similar to our findings. In another research, Adsumelli (12) classified 50 pregnant women on the cesarean section randomly into SCD and control groups, each including 25 participants. They applied the standard fluid therapy technique and spinal anesthesia to all the subjects. According to the results, the MAP decreased by 52% of the patients in the SCD group and 92% of the subjects in the control group (P = 0.004). Moreover, both groups did not show any significant difference regarding diastolic and systolic blood pressure, pulse pressure, and heart rate (12), which is similar to our results. Panigrahi et al. (13) classified 100 C-sections into SCD and control groups under spinal anesthesia. They compared the average dose of ephedrine needed, hypotension drop, the mean blood loss value, and the sensory block amount in terms of both rate and duration. Hypotension drop was proven to be significantly different in both SCD and control groups, with 24% and 54%, respectively (P = 0.002). Also, the SCD and control groups received 10 mg and 15.3 mg ephedrine, respectively (P = 0.008), which is similar to our results. Moreover, the groups demonstrated no statistically meaningful difference in the onset time of hypotension drop. A comparison between the two groups also revealed that the mean blood loss value and the level of sensory block were almost similar (13).
Tyagi et al. (14) compared continuous pressure non-pneumatic anti-shock garment (NASG) and intermittent SCD with a control group for the prevention of post-spinal hypotension in 90 parturient women aged 18 - 35 years undergoing elective cesarean sections with spinal anesthesia. They were randomly assigned to be applied with NASG, SCD, or no device (n = 30 in each group). A standardized protocol was done for hydration and anesthetic techniques. The primary outcome was the incidence of hypotension. The secondary outcome measures were the median dose of ephedrine required, incidence of maternal nausea and vomiting, and neonatal Apgar scores. In groups NASG, SCD, and C, the incidence of hypotension was 60%, 83%, and 90%, respectively (P = 0.021), with a significant lower incidence of hypotension in group NASG than in group C (P < 0.001, odds ratio: 0.17, 95% confidence interval: 0.04 - 0.68). The median (interquartile range) dose of ephedrine required was significantly lower in group NASG than in groups SCD and C (P = 0.002, P < 0.001, respectively) (14). These results are consistent with our results. The incidence of maternal nausea and vomiting was similar between the three groups and occurred in all patients post-delivery. The neonatal Apgar score at 1 and 5 min remained similar between the three groups (14).
5.1. Conclusions
This study showed that SCD can reduce the extensive changes in diastolic blood pressure as an important hemodynamic parameter and the incidence of nausea vomiting. Thus, SCD can be used in spinal anesthesia care practices for the elective cesarean section.
5.2. Limitations and Future Perspective
Limitations of this study were the small sample size, using a single-center, and lack of the evaluation of neonatal outcomes such as blood gas. Thus, we suggest that further studies include larger people from various centers and different settings for evaluating the effect of SCD during the cesarean section on the neonatal outcome.