1. Background
Abdominoplasty is considered one of the most commonly performed cosmetic procedures (1). According to statistics of the International Society of Aesthetic Plastic Surgery, a total of 758,590 abdominoplasty was performed in the world in 2016. There is an increase of 72.95% in comparison with 2011, making it the fourth most common cosmetic procedure (2). Given the increasing number of abdominoplasty performed, the importance of understanding the possible complications and morbidity associated with the procedure is critical. One of these complications is postoperative pain. With adequate postoperative pain control, the patients met discharge criteria earlier, and this helps in shortening the hospital stay, improved overall patient satisfaction, and decreased hospital costs (3). Therefore, anesthesiologists should be aware of the severity of this problem and the drug agents used to prevent and treat postoperative pain. Traditionally, analgesia for abdominal wall surgery is provided either by systemic drugs, such as opioids, non-steroidal anti-inflammatory agents, α-2 agonists, and paracetamol, or by regional analgesia (4, 5). However, opioids, such as morphine and fentanyl, remain the mainstay of postoperative analgesic regimens for patients after abdominal wall surgery. The pain is not always fully relieved by such agents, and the patients can develop tolerance to them. The ever-increasing doses of opioids are not without adverse effects (6, 7). Alternative approaches, which reduce the requirement for potent opioids postoperative, are required. Recently, interest has been renewed for the use of N-methyl-D-aspartate (NMDA) receptor antagonists, such as ketamine, for the management of postoperative pain.
Ketamine is an antagonist at NMDA receptors. It abolishes peripheral afferent noxious stimulation and prevents central sensitization of nociceptors as shown in animal studies with the excellent analgesic property even in sub-anesthetic doses (8). Various recently published studies have discussed the analgesic effect of low-dose ketamine (9-13). However, in these studies, the time of procedure was relatively short, surgical stimulation was moderate, and the sample size was small.
2. Objectives
This study was done to evaluate the efficacy of intravenous low-dose ketamine infusion versus morphine infusion analgesia for pain reduction in abdominoplasty.
3. Methods
This double-blinded, randomized, controlled clinical trial was approved by the local ethical committee (code: NCT03664622).
3.1. Sample Size
Power analysis was performed using the unpaired t-test for independent samples with time for analgesic supplementation because it was the primary outcome variable in the present study. Previous studies showed that the standard deviation of the total amount of intraoperative analgesic supplementation was about 2.3 mg in the placebo group with a mean of 4 mg, and the standard deviation of the amount of intraoperative analgesic supplementation was 1.6 mg with a mean of 2.9 mg in the ketamine group. Considering the power of 0.95 and alpha error of 0.05, a minimum of 71 patients was calculated for each group. A total of 160 patients (allocated into two groups) were regarded to compensate for possible dropouts (14).
The number of patients screened was 180, of whom 20 cases were excluded, and 160 patients completed the study protocol. The study was conducted on 160 patients scheduled for an elective abdominoplasty under general anesthesia at a tertiary teaching hospital from September 2018 to December 2019. The inclusion criteria were the American Society of Anesthesiologists (ASA) physical status I or II and the age of 18 - 50 years.
Exclusion criteria were as follows: those who refused to participate, ASA physical status III and IV, patients younger than 18 years or older than 50 years old, super morbid obesity with body mass index (BMI) of 50, a history of epilepsy, a history of parenteral or oral analgesics within the last 24 h before initiation of operation, or those having an allergy to study agents.
After a full explanation about the intervention and the potential hazards, informed consent was signed by every patient. The patients were randomly assigned to two groups by a computer-generated random number table using Excel (Microsoft Corporation, Redmond, WA, USA). The computer randomly allocated patients into two groups: (1) Group K (n = 80): ketamine group; intravenous ketamine was administered at a loading dose of 0.15 mg.kg-1 followed by an infusion at a dose of 2 µg.kg-1.min-1 (15); (2) group M (n = 80): morphine group; a loading dose of 0.1 mg.kg-1 was given intravenously, followed by a continuous intravenous infusion at a dose of 20 µg.kg-1 h-1.
The sequence of treatment was concealed until the interventions were assigned. Three anesthetists were involved in the study.
The randomization was performed by a physician who was not involved in clinical care or data collection. The second one was responsible for the drug administration according to each allocated group. The third anesthetist, blinded to the procedure, recorded the data at baseline (15 min preoperatively), at regular time intervals of 15 min after skin incision, and at regular intervals of 30 min postoperatively. Patients and the third anesthetists were blinded to group allocation throughout the procedure.
3.2. Preoperative Assessment
Details of the procedure were explained to the patients, and they were instructed about the evaluation of postoperative pain. All the patients were assessed clinically and investigated for the exclusion of any of the criteria mentioned above. The preoperative laboratory investigations included complete blood count (CBC), prothrombin time and concentration (PT and PC), partial thromboplastin time (PTT), bleeding time (BT), clotting time (CT), liver function tests, and kidney function tests. The following parameters were recorded: patient’s age, BMI, mean arterial blood pressure, heart rate (HR), and oxygen saturation.
3.3. Intraoperative Management
On arrival at the operating theatre, venous access was established, and then noninvasive blood pressure, electrocardiography, and pulse oximetry were connected for monitoring the patients. The induction of anesthesia was performed with intravenous propofol (2 mg.kg-1) and fentanyl (1 µg.kg-1). When the loss of consciousness was confirmed, an intravenous dose of 0.5 mg.kg-1 of atracurium was administrated. Adequate muscle relaxation was confirmed using a peripheral nerve stimulator before endotracheal tube insertion, and the patient mechanically ventilated (keeping peak airway pressure < 25 mmHg and EtCO2 30- 35 mmHg). Anesthesia was maintained with isoflurane (MAC 1) and O2 50% with a total fresh gas flow of 3 L/min-1 , and then the infusion of the drugs was commenced 10 min before the surgical incision as follows: Ketamine group: intravenous ketamine was administered at a loading dose of 0.15 mg.kg-1 over 5 min followed by a continuous infusion at a dose of 2 µg.kg-1.min-1 until the end of the surgery. Morphine group: a loading dose of 0.1 mg.kg-1 was given intravenously 10 min and followed by a continuous infusion of morphine at 1 µg.kg-1 until the end of surgery. The saline infusion was added to ensure blindness.
Fentanyl bolus doses (0.5 µg.kg-1) were adjusted to keep the HR and mean arterial blood pressure within 20% of the pre-induction values. Atracurium maintenance was commenced 20 min after continuous infusion at a dose of 0.5 mg.kg-1.h-1. At the end of the surgery, atracurium infusion was stopped, and neuromuscular blockade was reversed using 0.04 mg.kg-1 of neostigmine and 0.01 mg.kg-1 of atropine. The endotracheal tube was removed, and then the patients were transferred to the recovery room, in which the pain visual analogue scale (VAS) scores of the studied groups were registered for 4 h postoperatively. A standard postoperative analgesia regimen was prescribed as a single bolus of fentanyl 0.5 µg.kg-1 when the VAS was ≥ 3 or when the patient suffered from pain in the assessment intervals. Intravenous metoclopramide 0.15 mg.kg-1 was prescribed for patients who complained of nausea or vomiting.
3.4. Primary Outcomes
The amount of fentanyl given intraoperatively and postoperatively (for 4 h after extubation).
Secondary outcome parameters:
1) The VAS for pain assessment.
2) The Ramsey scale for the assessment of patient sedation:
Anxious agitated and restless = 1 point
The patient is cooperative, oriented, and tranquil = 2 points.
Responding only to verbal commands = 3 points.
Quick response to light glabella tap or loud auditory stimulus = 4 points.
Sluggish response to light glabella tap or loud auditory response = 5 points.
No response to light glabella tap or loud auditory response = 6 points.
The anticipated side effects of ketamine, like sedation, hallucinations, headache, nausea, vomiting, and drowsiness were observed.
3.5. Statistical Analysis
Statistical analysis was performed using SigmaStat software version 3.1 (Systat Software, Inc., Point Richmond, CA, USA). Patients' data were collected and presented as mean ± SD or median (interquartile range), as appreciate. The analysis was carried out using the unpaired student's t-test between the two groups and the Chi-square test for dichotomous data.
4. Results
The number of patients screened was 180, and after excluding 20 cases, 160 patients completed the study protocol (Figure 1). There were no statistically significant differences between the two studied groups regarding age, BMI, co-morbidities, and surgical time (Table 1).
| Group (K) | Group (M) | P-Value | |
|---|---|---|---|
| Age, y | 33.03 ± 6.14 | 32.23 ± 5.21 | 0.37 |
| BMI, kg/m2 | 38.51 ± 6.48 | 37.1 ± 6.7 | 0.12 |
| CO-morbidities | |||
| Hypertension | 10 (12.5) | 14 (17.5) | 0.41 |
| Diabetic | 9 (11.25) | 7 (8.75) | |
| Asthmatic | 12 (15) | 8 (10) | |
| Ischemic heart disease | 0 (00) | 2 (2.5) | |
| Surgical time, min | 236.2 ± 58.32 | 246.63 ± 60.96 | 0.27 |
| Intraoperative fentanyl requirement, µg | 133.1 ± 39.5 | 125 ± 33. 5 | 0.08 |
| Postoperative fentanyl requirement, µg | 109.06 ± 42.9 | 99.06 ± 27.1 | 0.08 |
| Sedation | 3/80 | 8/80 | 0.091 |
| Hallucinations | 6/80 | 2/80 | 0.0910 |
| Nausea and vomiting | 4/80 | 9/80 | 0.0911 |
Abbreviations: K, ketamine group; M, morphine group.
aValues are expressed as mean ± SD, No. (%).
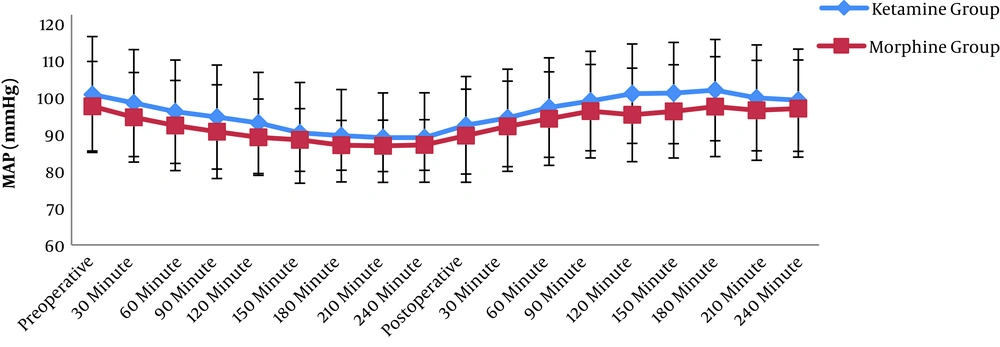
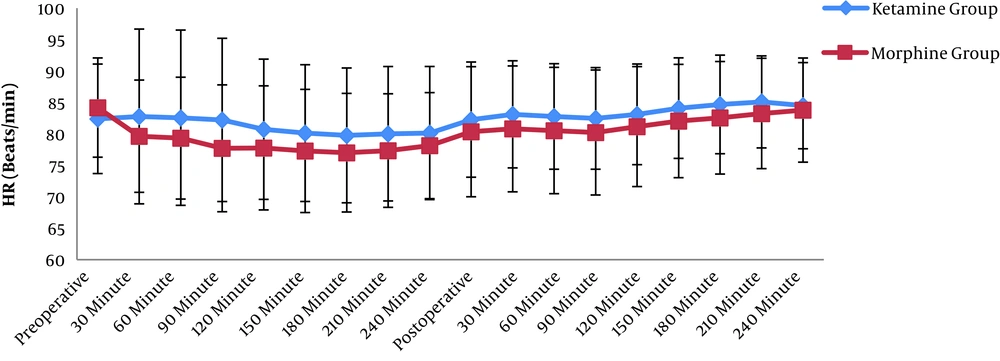
The patients’ mean arterial blood pressures and HR were recorded intraoperatively at an interval of 30 min. The same was performed postoperatively every 30 min, for a total time of 4 h, and no statistically significant difference was detected (Figures 2 and 3).
Regarding fentanyl rescue doses, there was no statistically significant difference as it was 133.1 ± 39.5 µgin group K, and 125 ± 33.5 µgin group M (P-value = 0.08). Likewise, the rescue doses postoperatively showed no statistically significant difference as it was 109.06 ± 42.9 µgin K group and 99.06 ± 27.1 µgin group M (P-value = 0.08) (Table 1).
The Ramsay score and VAS were evaluated postoperatively every 30 min for a total time of 4 h. The Ramsay score showed no statistically significant difference before 150 min postoperatively. However, it was significantly lower in group K compared with group M after 150 min postoperatively. On the other side, the VAS was significantly higher in group K compared with group M (Tables 2 and 3). In addition to the previous results, there were no remarkable differences between both groups concerning the incidence of complications, such as sedation, nausea, vomiting, and hallucinations (Tables 1 and 3).
| Group (K) | Group (M) | P-Value | |
|---|---|---|---|
| Postoperative | 4 (1 - 5) | 3 (1 - 5) | 0.14 |
| After 30 min | 4 (1 - 5) | 3 (2 - 5) | 0.19 |
| After 60 min | 4 (1 - 5) | 3 (1 - 5) | 0.14 |
| After 90 min | 4 (2- 5) | 3 (1 - 5) | 0.14 |
| After 120 min | 3 (1 - 5) | 3 (2 - 4) | 0.15 |
| After 150 min | 4 (1 - 5)b | 3 (1 - 4) | 0.03 |
| After 180 min | 4 (3 - 7)b | 3 (2 - 5) | < 0.05 |
| After 210 min | 4 (3 - 8)b | 3 (2 - 5) | < 0.05 |
| After 240 min | 4 (2 - 8)b | 4 (2 - 4) | < 0.05 |
Abbreviations: K, ketamine group; M, morphine group.
aValues are expressed as median (range).
bP-value < 0.05.
| Group (K) | Group (M) | P-Value | |
|---|---|---|---|
| Postoperative | 2(2 - 4)b | 3 (2 - 5) | < 0.05 |
| After 30 min | 2(2 - 4)b | 3 (2 - 5) | < 0.05 |
| After 60 min | 2 (1- 4)b | 2 (2 - 4) | < 0.05 |
| After 90 min | 2 (1 - 2)b | 2 (2 - 3) | < 0.05 |
| After 120 min | 2 (1 - 2)b | 2 (2 - 3) | < 0.05 |
| After 150 min | 2 (1 - 2) | 2 (1 - 3) | 0.06 |
| After 180 min | 2 (1 - 2) | 2 (1 -3) | 0.31 |
| After 210 min | 2 (1 - 2) | 2 (1 - 3) | 0.126 |
| After 240 min | 2 (1 - 2) | 2 (1 - 3) | 0.7 |
Abbreviations: K, ketamine group; M, morphine group.
aValues are expressed as median (range).
bP-value < 0.05.
5. Discussion
Intraoperative analgesia is traditionally managed by opioids. There is a need for an adjuvant or alternative to opioids to reduce their usage due to their potential hazards, especially respiratory depression. Low-dose ketamine (< 1 mg.kg-1), with sub-dissociative effect, presents a desirable alternative as it has a low incidence of respiratory depression and is a commonly used medication for a long time in the usual anesthetic practice.
In this study, ketamine showed a practical analgesic effect similar to morphine in abdominoplasty. This analgesic effect is equivalent to morphine and fentanyl. However, it failed to reduce the fentanyl consumption in the 4 h postoperatively and showed no statistically significant differences regarding side effects between the groups.
Ketamine has been tested in many studies to examine its analgesic effect either preoperatively or intraoperatively (9-17). Minoshima et al. (14) examined a low dose of ketamine infusion 48 h intra- and postoperatively versus the placebo in 36 patients subjected to scoliosis corrective surgery and found that Ketamine infusion reduced morphine consumption significantly over the next 48 h.
Tseng et al. (16) emphasized the results of our study as they examined a combination of fentanyl and low-dose ketamine as patient-controlled analgesia (PCA) versus epidural PCA in 70 patients after video-assisted thoracic surgery. Both groups were monitored for 24 h and showed no differences regarding pain profile (VAS), and there were no statistically significant differences between both groups in the rescue doses of analgesics.
Moreover, Bilgen et al. (17) used three different doses of ketamine as a bolus with the induction of anesthetics in cesarean sections and monitored the morphine consumption for the next 48 h. They found no difference between the groups.
Other authors published reviews of randomized controlled trials testing ketamine in the emergency department. These reviews showed that low-dose ketamine is effective for pain relief in the emergency department. It is not superior to other opioids but has a safer profile as it can be used safely by the nursing staff and has no risk for addiction or respiratory depression. Moreover, it has a minimal incidence of delirium or hallucinations. These reviews support the results of this study as ketamine was used in the concise painful procedure and the form of a bolus. The results were again not superior but equivalent to the opioids with the benefit of safety and cost of delirium (18, 19).
Jouguelet-Lacoste et al. (20) reviewed 39 clinical trials, in which it was found that continuous infusion is superior to boluses, and infusion of ketamine could reduce opioid consumption in the postoperative period by 40%. In this meta-analysis, ketamine as an IV bolus dose of less than 1.2 mg.kg-1.h-1 was considered. This result was astonishing and it was not similar to the previous studies.
Nevertheless, Brain et al. may explain this result. They did a narrative review to assess the best dose and method of administration, and they found that a bolus followed with intra-operative infusion for 48 h is the best way to reduce postoperative pain.
Moreover, the effect was dose-dependent and limited by the side effects. This review recommends a continuous ketamine infusion for 48 h to have an effective reduction in opioid consumption, which was not done in the mentioned studies (21).
Lastly, ketamine is not a perfect drug; it has many complications, such as hallucinations, delirium, nightmares, sleep disturbance, nausea, and vomiting. Although all of these side effects were not examined in this study, some of them were seen during the short postoperative follow-up, such as nausea, vomiting, hallucinations, and delirium. It was clear that side effects and complications are related to the dose (22, 23).
5.1. Limitations
This study had a limitation because of a short follow-up time as the patients were followed only for 4 h in the recovery room.
5.2. Conclusions
Low-dose ketamine has a useful analgesic effect in abdominoplasty similar to morphine without remarkable side effects.
Informed consent was signed by every patient.