1. Context
Lumbar herniated nucleus pulposus (HNP) is one of the most common causes of low back pain (LBP), where nearly 80% of people worldwide experience at least one LBP episode during their lifetime, and it causes disability. The prevalence of lumbar HNP ranges from 1% to 3% of the population worldwide. Thus, it is required to re-evaluate patients during follow-ups for diagnostic and specific therapy (1).
Disc herniation can directly or indirectly trigger a pain response. Directly, disc herniation can cause ganglion compression, stimulating nociceptors in the ligament and annulus fibrosus through stretching, which causes direct deformity or traction of intrathecal nerve fibers and nerve roots. Indirectly, disc herniation can cause ischemia due to compression of vascular nerve fibers, which leads to venous stasis with changes in venous reflux and, consequently, edema and trophic changes in nerve fibers (1, 2).
Conservative therapy (pharmacotherapy or physiotherapy) is effective in 60% of cases, while in other cases continues to become chronic pain and results in a high degree of disability and higher medical costs (1). Surgical intervention with good results usually has more risks, such as neurological trauma, slow recovery time, spinal instability, adhesions, scarring, and even surgical failure in severe cases. Therefore, currently, minimally invasive pain treatment techniques are being developed due to their low-risk complication (2).
Radiofrequency therapy is a medical procedure mainly used to reduce pain with a low complication rate (less than 1%), ease of application, and low cost (1). The electric current generated by radio waves is used to heat a small nerve tissue area, thereby inhibiting or reducing pain signals from that particular area. In the last five years, radiofrequency has been developed for diseases related to the spinal functional unit (1, 3-5).
Research results regarding the PRF effectiveness as a modality of pain therapy are mixed, and systematic reviews of PRF are still limited. Therefore, research, especially randomized controlled trials (RCTs), is needed to support scientific evidence of the PRF effectiveness in managing radicular pain in lumbar HNP.
This study aimed to systematically review the PRF effectiveness in treating radicular pain in patients with lumbar HNP and to evaluate the PRF procedure’s safety in managing radicular pain in lumbar HNP.
2. Methods
2.1. Literature Search and Study Selection
The literature search for this systematic review was carried out using the preferred reporting items for systematic reviews and meta-analyses reporting guidelines, and the literature involved in the review was articles published until August 1, 2020. An online search was conducted on some electronic databases, such as PUBMED, SCOPUS, Cochrane Library, and Science Direct. The search terms “radiofrequency” and “radicular pain” or “herniated nucleus pulposus” were used.
2.2. Eligibility Criteria
Inclusion criteria were original research written in English and research aimed at determining the pulsed radiofrequency (PRF) effect on radicular pain in lumbar HNP, which contained information including:
1) Population: Human studies and subjects diagnosed with lumbar HNP with radicular pain, with both sexes being above 18 years of age and below 65 years of age.
2) Intervention: PRF.
3) Comparisons: Standard radicular pain therapy in lumbar HNP, placebo, and other pain interventions (such as continuous radiofrequency and transforaminal steroid epidural injection).
4) Outcome: The PRF effectiveness (the decreased VAS score) and the presence or absence of side effects during and after the treatment.
5) Study design: RCT.
Non-English articles, duplicate articles, literature reviews, studies on cadaveric samples, laboratory studies, animal studies, biomechanical studies, letters to editors, instructional courses, and technical notes were excluded. We also excluded articles with incomplete information on diagnosis, examination, follow-up duration, postoperative clinical outcomes, and no statistical analysis.
2.3. Study Screening and Data Extraction
Two authors (A.M and S.R) independently screened the study and extracted data. Disagreements were resolved by discussion between two review authors. If no agreement could be reached, consultation with a third author would be performed. The reference lists of all the included studies were screened for additional articles relevant to this review.
Data were extracted from the articles using a predesigned form, including articles, name of journal or conference, year, topic, title, participants, keywords, country, research methodology, pain scale before and after the intervention, and control. The authors assess potentially relevant articles. The assessment consisted of reading the full text and extracting the reviewed data.
2.4. Quality of Assessment and Risk of Bias
The reliability of randomized trial results depends on the extent to which potential sources of bias are avoided. The risk assessment of bias in each journal was performed using the Cochrane handbook of systematic reviews of interventions.
Proper classifications of bias are selection bias, performance bias, attrition bias, detection bias, and reporting bias. The literature was assessed using the questionnaire of the critical review worksheet from the University of Oxford Center for Evidence-Based Medicine (CEBM) downloaded at www.cebm.net on July 28, 2020.
2.5. Statistical Analysis
The mean SD with a 95% confidence interval (CI) was used to evaluate the difference in pain reduction (VAS) scores between the PRF and control groups with different monitoring times (four, eight, and 12 weeks after treatment). The heterogeneity between the studies was evaluated using statistical analysis, where P < 0.05 indicated statistically significant heterogeneity. Unfavorable data were analyzed using the random-effects model approach. The heterogeneity in the initial data was explored with sub-group analysis based on various variables, such as study location, PRF technique, sex, and concurrent therapy. Publication bias was assessed using a funnel plot graph. All data analyses and presentations were performed using Review Manager version 5.3.
3. Results
3.1. Study Selection and Characteristic
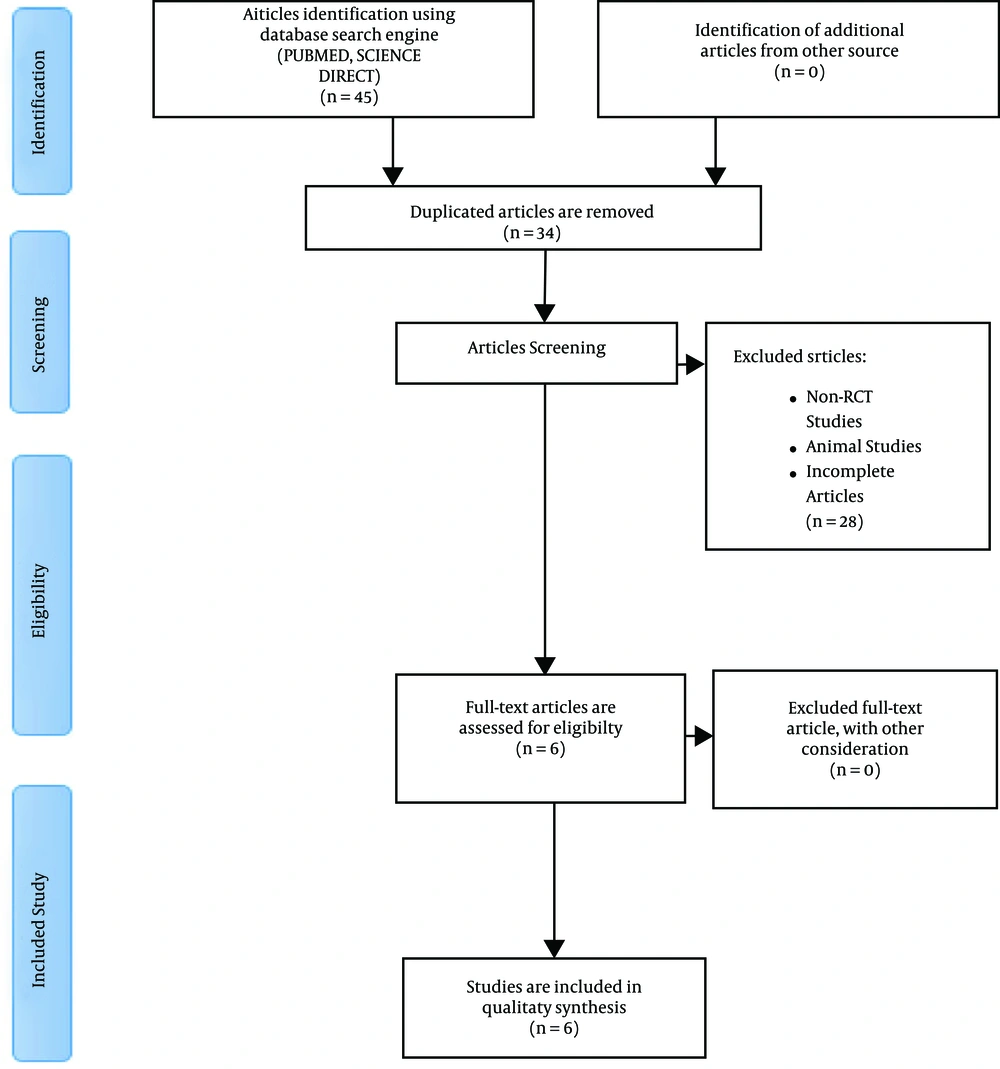
Forty-five articles were obtained from the database literature search, and 11 articles were excluded based on their titles or due to duplication. Finally, a total of 34 articles were eligible for further screening, of which 28 were excluded because they did not match the inclusion criteria. Thus, six full articles were included in this review. The flowchart of articles selected for the review is provided in Figure 1. All the included studies were randomized controlled trials by design.
Some demographic data were obtained from the six studies that met the inclusion and exclusion criteria in this systematic review. The mean age range was between 37 and 62 years in the six studies. Each study’s inclusion criteria provided an age limit for subjects between 18 and 65 years, and the sex ratio varied in the six studies. Males had a more significant number than females, except in Lee et al.’s study (6), where the number of women was more than men. The duration of radicular pain in the six studies was more than three months. The most dominant HNP level in the six studies was in the L5-S1 segment (Tables 1 and 2).
| Main Author (Year) | Country | Sample Size | Male: Female | Age, Mean ± SD | Duration of Pain, mo | Measurement of Clinical Outcomes | Level of Dominant HNP | Previous Pain Score, Mean ± SD | Quality of the Study (OCEBM) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PRF Group | Control Group | PRF Group | Control Group | PRF Group | Control Group | |||||||
| Simopoulos et al. (2008) (7) | United States of America | 76 | 45:31 | 39 | 55.1 ± 14.3 | 53.8 ± 14 | > 6 | VAS | L5-S1 | 7.8 ± 1,6 | 7,1 ± 1,9 | 2 |
| Koh et al. (2012) (8) | South Korea | 62 | 11:20 | 10:21 | 65.97 ± 7.25 | 65.16 ± 8.96 | ≥ 3 | NRS; ODI | L5 | 7.39 ± 0.37 | 7.00 ± 0.43 | 2 |
| Shanthanna et al. (2014) (9) | Canada | 31 | 10:6 | 8:7 | 57 (35 - 83) | 57 (35 - 83) | ≥ 4 | VAS | L5-S1 | not reported | not reported | 2 |
| Lee et al. (2015) (6) | South Korea | 18 | Not reported | Not reported | 54.3 ± 12.1 | 50.8 ± 12.7 | 5 | VAS; ODI | L5-S1 | 5.3 ± 1.2 | 4.9 ± 0.8 | 2 |
| Khalifa and Saadalla (2017) (10) | Egypt | 90 | 59:31 | 45 | 37.5 ± 9 | 39.3 ± 8.8 | > 3 | VAS | L5-S1 | 7.53 ± 0.5 | 7.5 ± 0.5 | 2 |
| De et al. (2018) (11) | India | 50 | 9:16 | 13:12 | 41.92 ± 14.53 | 41.4 ± 10.64 | > 3 | VAS; ODI | L5-S1 | 8.24 ± 0.96 | 8.12 ± 1.05 | 2 |
Characteristics of Each Study
| Author (Year) | Therapeutic Target | Intervention Parameter | Control Group | Follow up | Result | Adverse Effect | Result of the Study | Limitation | |
|---|---|---|---|---|---|---|---|---|---|
| Pain | Functional | ||||||||
| Simopoulos et al. (2008) (7) | DRG | 42°C, 120 seconds | PRF + CRF | Eight weeks; eight months | Significant compared to the control group | not reported | not reported | 70% of the PRF group patients and 82% of the PRF + CRF group patients had a reduced pain intensity in the second month. The analgesic response was 3.18 months (± 2.81) in the PRF group and 4.39 months (± 3.50) in the PRF + CRF group. The chances of success for both groups were close to 50% in the third month. After eight months, most of the patients experienced no improvement in pain. There were no statistical differences between the PRF group and the PRF + CRF group. | Short-term evaluations were not reported; side effects were not reported. |
| Koh et al. (2012) (8) | DRG | 42°C, 120 seconds | Sham (needle placement) | Four weeks; eight weeks; 12 weeks | Significant compared to the control group | Not Significant compared to the control group | No side effect | The number of patients with successful treatment outcomes was higher in the PRF group in the second (P = 0.032) and third months (P = 0.018). No significant differences were observed regarding the secondary outcome variable (ODI) between the two groups. | Long-term evaluations were not reported. |
| Shanthanna et al. (2014) (9) | DRG | 42°C, 120 seconds | Sham (needle placement) | 24 hours; one week; Four weeks; eight weeks; 12 weeks | Not Significant compared to the control group | Not Significant compared to the control group | No side effect | The differences in VAS and ODI reduction in the PRF groups were not significant in the fourth week and the third month compared to the placebo (oral analgesic drug). Six out of 16 subjects in the PRF group and three out of 15 subjects in the control group experienced a VAS reduction of > 50%. There were no PRF side effects. | Small sample size; no initial VAS data was included; long term evaluation was not reported. |
| Lee et al. (2015) (6) | DRG | 42°C, 240 seconds | TFESI (dexamethasone 5 mg | Two weeks; Four weeks; eight weeks; 12 weeks | Not Significant compared to the control group | Not Significant compared to the control group | increasing of radicular pain (n = 1) | The difference in VAS reduction, ODI in the PRF group was not statistically significant compared to the control group (TFESI of dexamethasone 5 mg) | Small sample size; low baseline VAS; long term evaluations were not reported. |
| Khalifa and Saadalla (2017) (10) | DRG | 42°C, 120 seconds | TFESI (methylprednisolone 2 mg) | One week; Four weeks; eight weeks; 12 weeks | Significant compared to the control group | Significant compared to control group | not reported | The difference in VAS reduction, ODI (dorsal lumbar root ganglion) was more significant in the PRF group than in the control group (transforaminal epidural injection of methylprednisolone steroid 24 mg) up to three months of follow-up in lumbar HNP patients. | Long-term evaluations were not reported; side effects were not reported |
| Mohan et al. (2018) (11) | DRG | 42°C, 180 seconds | TFESI | Two weeks; Four weeks; Eight weeks; 12 weeks; 24 weeks | Significant compared to the control group | Significant compared to control group | not reported | The difference in VAS reduction, ODI (dorsal lumbar root ganglion) was more significant in the PRF group than in the control group (TFESI injection) up to 24- week evaluation. | Long-term evaluations were not reported; side effects were not reported. |
Parameters of the Intervention Procedure, Outcome, Side Effects, and Limitations of Each Study
3.2. Included Study
The RCT study conducted by Simopoulus et al. (7) aimed to compare the effects of PRF and controls on lumbar HNP radicular pain (PRF plus continuous radiofrequency/CRF).
A total of 76 subjects with chronic lumbosacral radicular pain refractory to conventional therapy lasting > 6 months meeting the inclusion criteria were randomly assigned to two intervention groups: a PRF group and a PRF plus CRF group. In the first group, subjects received PRF with a temperature of 42°C for 120 seconds, while in the second group, subjects received PRF with a temperature of 42°C for 120 seconds, and then CRF with a temperature of 54oC ± 5 for 60 seconds. The intervention response was evaluated in the second month, and then re-evaluation was performed every month (7).
Two months after the procedure, 70% of the PRF group patients and 82% of the PRF + CRF group patients experienced a reduction in pain intensity. The mean duration of a successful analgesic response was 3.18 months (± 2.81) in the PRF group and 4.39 months (± 3.50) in the PRF + CRF group. Kaplan-Meier’s analysis illustrated that both groups’ chances of success were close to 50% in the third month (7). This study concludes that PRF in the dorsal root ganglion (DRG) is a safe pain therapy for chronic lumbosacral radicular pain. A large number of patients can obtain at least a short-term benefit. The addition of heat via CRF did not provide a significant advantage (7).
Koh et al. (8), in their study, aimed to determine the PRF effect on chronic lumbosacral radicular pain compared to a control group with a total of 62 subjects. Two and three months after the treatment, it was found that the numerical rating scale (NRS) pain score was more significantly reduced in the intervention group than in control with P = 0.032, and P = 0.018, respectively.
This study concludes that PRF can be applied in combination with a transforaminal epidural steroid injection (TFESI) to obtain higher therapeutic effectiveness compared to TFESI alone (8).
Shanthanna et al. (9), in their study, aimed to determine the PRF effect on lumbar HNP radicular pain. In this triple-blind, single-center, placebo-controlled, RCT study, patients were randomized into a placebo (sham) group and a treatment group using PRF on DRG at 42°C for 120 seconds. The patients were evaluated for three months after the procedure. Outcomes related to VAS, ODI, and side effects were analyzed based on the intention-to-treat analysis.
The difference in the average VAS did not differ significantly in the fourth week or the third month. Similarly, the Oswestry disability index (ODI) scores did not differ significantly in the fourth week or the third month. There was no severe side effect during the study. Six out of the 16 patients in the PRF group and three out of the 15 patients in the placebo group showed a 50% reduction in VAS scores (9). This study concludes that the PRF-DRG success rate is relatively low in the third month post-treatment (9).
Lee et al. (6) compared the effects of PRF and TFESI. The inclusion criteria were subjects with radicular pain due to lumbar HNP, with VAS > 4 and ODI > 30% (after the first TFESI injection with 2 mL 0.125% bupivacaine combined with 5 mg dexamethasone). The RCT study design, without any blinding method, included 18 subjects, consisting of 10 subjects in the PRF group and eight subjects in the control group. The PRF group was given PRF therapy with a temperature not exceeding 42°C for 240 seconds. The TFESI group was given an injection of 2 mL of 0.125% bupivacaine combined with 5 mg of dexamethasone. The results showed that the mean VAS score for radicular pain and the ODI score was significantly lower 12 weeks after treatment in both study groups. However, no significant differences were observed between the PRF and TFESI groups.
This study concludes that PRF is considered an applicable clinical option to control subacute radicular pain, reducing or avoiding the possible adverse catastrophic side effects of TFESI (6).
Khalifa and Saadalla (10), in their RCT study, compared the effects of PRF on DRG and TFESI intervention on radicular pain therapy due to lumbar HNP. A total of 90 subjects were randomly allocated into two groups: The TFESI group with 45 subjects and the PRF group with 45 subjects. In the TFESI group, methylprednisolone was injected via a transforaminal epidural into the affected roots at a dose of 24 mg/root. Meanwhile, the PRF group was subjected to PRF on DRG at a temperature of 42°C for 4 minutes (240 seconds), and only 8 mg of methylprednisolone was injected after PRF intervention. A doctor assessed the intervention effectiveness by comparing the VAS score before the treatment, as well as one week, four weeks, two months, and three months after the treatment via a telephone call.
In the first week, the VAS score was significantly reduced in both groups, and the difference was not significant between the two groups (P = 0.052). In the fourth week, the VAS score reduction was still significant in both groups, but the reduction was more significant in the PRF group than in the TFESI group (P < 0.001). In the second month, there was no significant difference between pre-intervention and post-intervention VAS scores in the TFESI group, whereas the post-intervention VAS score was significantly better than the pre-intervention VAS score in the second month in the TFESI group (P < 0.001). In the third month, the decrease in the VAS scores was more significant in the PRF group than in the TFESI group (P < 0.001) (10).
This study concludes that PRF in DRG is more effective than a lumbar TFESI injection up to a three-month follow-up in patients with radiculopathy due to lumbar disc prolapse (10).
Mohan et al. (11) compared the effects of PRF and TFESI on lumbosacral radicular pain. A total of 50 samples were randomly allocated to a PRF group (n = 25) and a control group (n = 25). There was a significant decrease in VAS and ODI scores in the PRF group compared to controls two weeks, one month, two months, three months, and six months after the treatment.
3.3. Risk of Bias Assessment
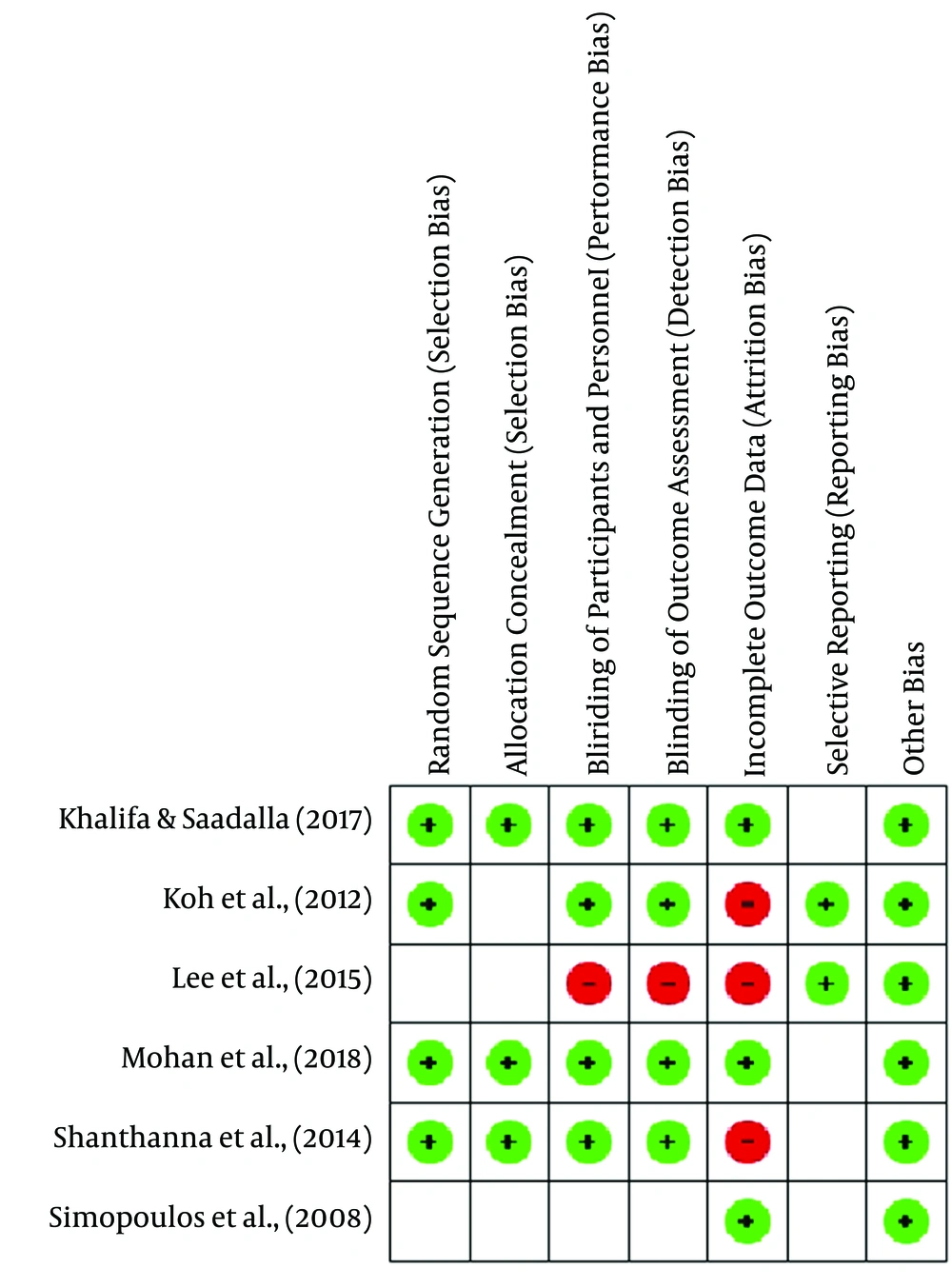
The risk of bias summary and graph is presented in Figures 2 and 3.
3.3.1. Random Sequence Generation
Khalifa and Saadalla (10) and Shanthanna et al. (9) showed that the randomization technique was carried out by the sealed envelope method. Koh et al. (8) and Mohan et al. (11) used computer-based randomization and were categorized as low-risk bias. Meanwhile, other studies did not mention the randomization method and were assessed as unclear risk of bias.
3.3.2. Allocation Concealment
Khalifa and Saadalla (10), Shanthanna et al. (9), and Morgan et al. implemented allocation concealment using the sealed envelope method, and thus they were rated as low risk of bias. However, other studies did not apply allocation concealment and thus were assessed as unclear risk of bias.
3.3.3. Blinding of Participants and Personnel
Khalifa and Saadalla (10), Koh et al. (8), Shanthanna et al. (9), and Morgan et al. (2018) used a double blindness procedure among subjects and examiners, and thus, they were rated as low risk of bias. In contrast, Lee et al. (6) did not use the blinding technique, and thus they were considered to have a high risk of bias.
3.3.4. Blinding of Outcome Assessment
The risk of detection bias is considered low if subjects providing an assessment of the intervention outcome are selected using the blinding technique. Thus, the studies of Khalifa and Saadalla (10), Koh et al. (8), and Shanthanna et al. (9) were assessed as low risk of bias. Meanwhile, the studies of Lee et al. (6) and Simopous et al. (7) were assessed as high risk of bias.
3.3.5. Incomplete Outcome Data
Shanthanna et al. (9) stated that three subjects were lost to follow-up during the third month of evaluation. Koh et al. (8) stated that there were missing data at follow-up at each evaluation stage (seven subjects in the fourth week, 10 subjects in the eighth week, and 13 subjects in the twelfth week). Thus, the studies were rated as high risk of bias.
3.3.6. Selective Reporting
Lee et al. (6) and Koh et al. (8) included the research protocol registration number in their studies, and thus they were rated as low risk of bias. However, other studies did not include this number and were assessed as unclear risk of bias.
3.3.7. Other Potential Sources of Bias
We did not identify other sources of bias. Thus, all the studies were rated as low risk of bias.
3.4. Evaluation of Publication Bias
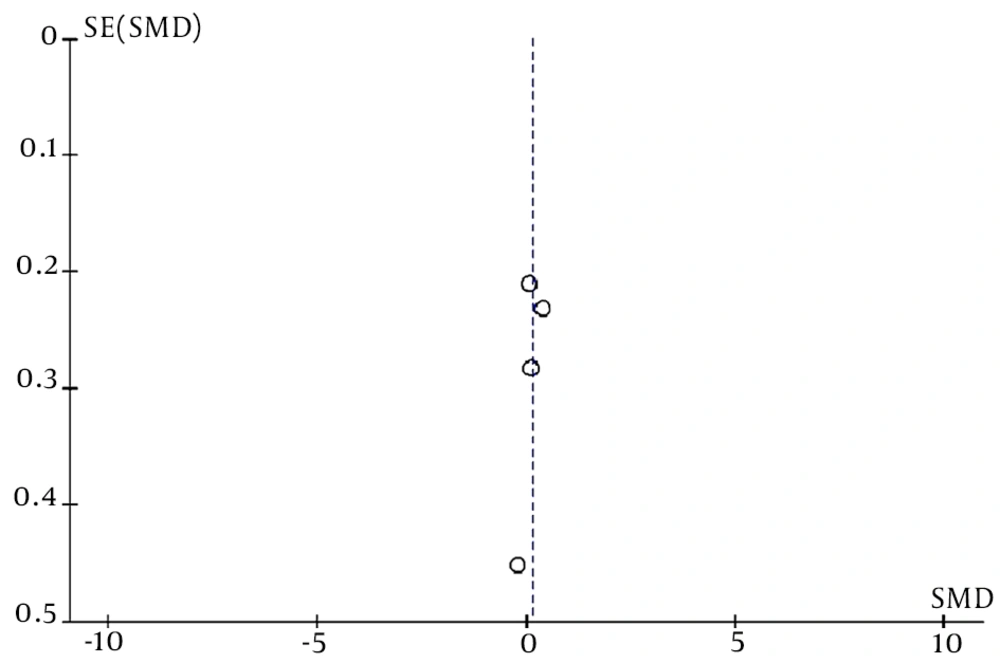
Publication bias in this meta-analysis was evaluated using a funnel plot chart. A funnel plot is a graph that presents the extent of the effect of the sample size of a study (y-axis) on its results (x-axis). A higher bias leads to more unequal data and less homogeneous data. As shown in Figure 4, the data are not scattered, suggesting that this meta-analysis contained no publication bias.
3.5. Result of Critical Analysis and Meta-analysis
3.5.1. Initial Pain Score
Five of the studies used the same pain measurement scale, which is VAS, and the remaining one used NRS. The average VAS score at baseline before action was > 4, with the lowest VAS score (5.3 ± 1.2) obtained among subjects in Lee et al.’s study. The homogeneity of the initial data in the meta-analysis revealed homogeneous data (P = 0.23), and there was no significant difference between the initial VAS scores (6).
3.5.2. PRF Effectiveness
The six studies showed that pain scores at each evaluation were significantly reduced in the PRF group (the fourth, eighth, and twelfth weeks). Compared to the control (TFESI), four of the studies showed that the PRF group had a more significant reduction in pain scores (7, 8, 10, 11). However, Lee et al. (6) and Shanthanna et al. (9) in their studies showed that the reduction in pain scores was not significantly higher in the PRF group than in control (PRF or TFESI).
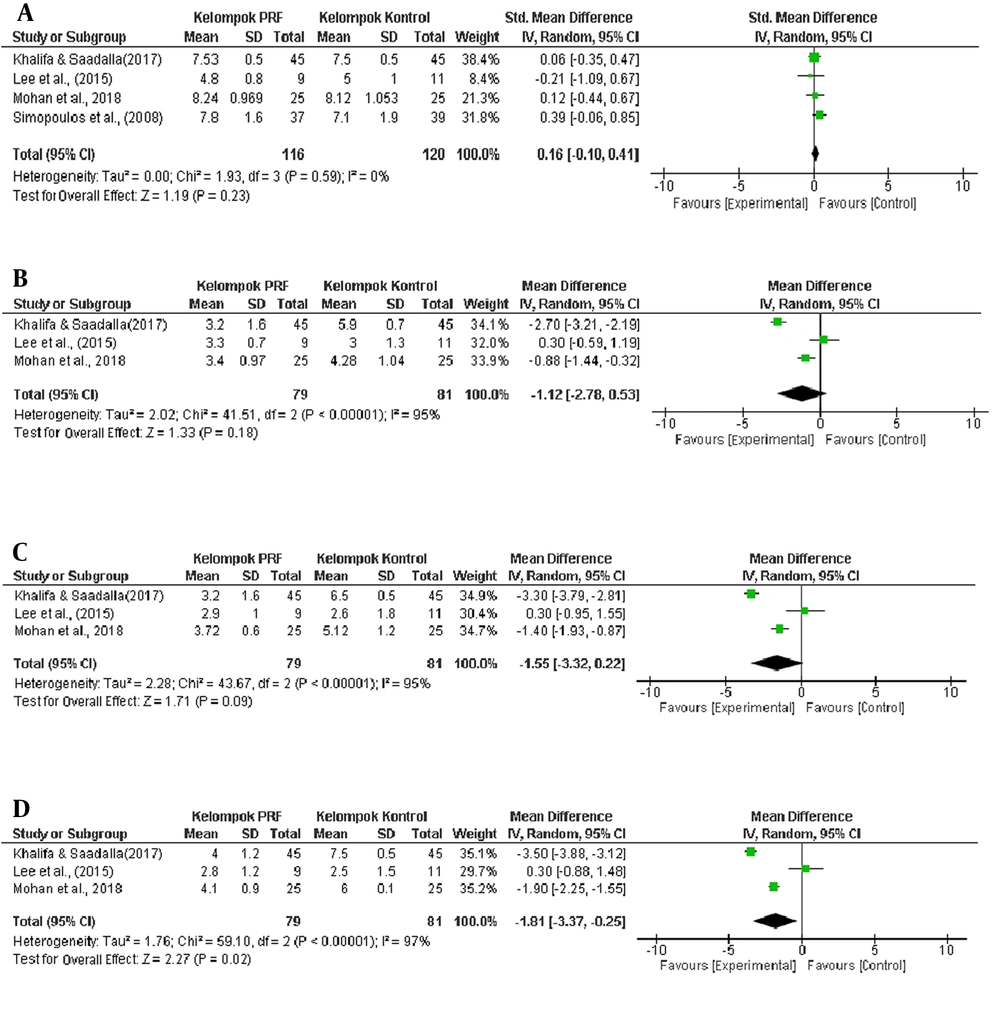
The addition of CRF therapy did not create a more significant effect than that of PRF alone in the PRF group (7). The statistical analysis revealed that in the fourth- and eighth-week evaluation, the decrease in VAS was not significant in the PRF group that in the control group (P = 0.18 in the fourth week and P = 0.09 in the eighth week). A significant reduction in VAS in the PRF group than the control group occurred in the 12th-week evaluation (P = 0.02), as explained in the forest plot graph (Figure 5).
3.5.3. Adverse Effect
Koh et al. (8) and Shanthanna et al. (9) in their studies found no side effects during and after PRF treatment, including post-treatment pain, allergies, or neurological deficits caused by PRF. Meanwhile, Lee et al. (6) found one subject who experienced increased radicular pain after PRF action and then withdrew from the study. Moreover, Simopoulos et al. (7), Khalifa and Saadalla (10), and De et al. (11) did not report side effects in their studies.
4. Discussion
This study involved six RCTs and 327 participants in evaluating the PRF effect on radicular pain in lumbar HNP. All the studies were assessed systematically. However, not all the studies were included in the meta-analysis. The reason is limited data, where there were differences in the size of the clinical outcome, time of evaluation (follow-up), and incomplete data for statistical analysis. Only studies with a VAS outcome and evaluations in the fourth, eighth, and twelfth weeks could be statistically analyzed.
The PRF effectiveness, as measured by decreasing the VAS score, showed a significant pain score reduction from baseline to the last evaluation, by which the pain score was reduced by ≥ 50% from the baseline pain score. However, only four of the studies showed statistically significant results when compared to controls, including the studies by Simopoulus et al. (7), Koh et al. (8), Khalifah and Saadalla (10), and De et al. (11). Meanwhile, the remaining two studies, Shanthanna et al. (9) and Lee et al. (6), did not show significant results.
The statistical analysis found that in the fourth- and eighth-week evaluation, the decrease in VAS in the PRF group was not significant compared to the control group (P = 0.18 at week four and P = 0.09 at week eight). A significant reduction in VAS in the PRF group compared to the control group occurred in the 12th-week evaluation (P = 0.02), as explained in the forest plot graph (Figure 5).
The mechanism of action of PRF in pain management (in this case, radicular pain in lumbar HNP) is currently not known with certainty. Radiofrequency is believed to have neuromodulatory and anti-inflammatory effects. Microscopic damage is found after exposure to radio waves, as observed in membrane abnormalities and mitochondrial morphology, as well as in interruption and disorganization of microfilaments and microtubules. This ultrastructural pathway occurs more widely in type C and type A nerve fibers, which are the main nociceptors. Radio waves affect immune cells and inhibit the production of pro-inflammatory cytokines, such as interleukin-1b and interleukin-6 (1, 2).
Several studies have also shown that radiofrequency can induce changes in membranes and intracellular structures, thereby modifying the transmission of action potentials and perception of pain (1, 3, 12-14).
Erdine et al. (15) evaluated ultrastructural lesions on sensory nociceptive axons occurring after PRF intervention using an electron microscope. They asserted that PRF action selectively produced a wider range of lesions in smaller primary sensory nociceptors, such as Aδ and C fibers, compared to larger non-pain sensory fibers (15). PRF activates the descending noradrenergic and serotoninergic pain inhibition pathways and inhibits excitatory nociceptive C fibers (16).
PRF intervention in DRG can decrease microglial activity in the spinal cord’s dorsal horn. Decreased microglial activity may prevent the development of chronic neuropathic pain, where microglia can cause chronic neuropathic pain through the release of various cytokines and chemokines associated with the transmission of pain signals (17). Non-inflammatory cytokines, such as tumor necrosis factor-α and interleukin-6, decrease after PRF intervention (18).
Research conducted on mice’s sciatic nerve 10 days after the PRF treatment showed the physical evidence of ultrastructural damage after PRF exposure to nociceptive axons of sensory nerve fibers. Mitochondria, microtubules, and microfilaments all exhibit varying degrees of damage and disturbance. The damage increases progressively from Aβ nerve fibers to Aδ nerve fibers and to C nerve fibers, consistent with quantitative calculations of the PRF electric field’s strength penetrating inner axons. It is also consistent with the idea that PRF has a more significant selective effect on pain-carrying nerve fibers smaller in diameter (nerve fibers C and Aδ), but less effect on larger nerve fibers (15).
The lack of data required for statistical analysis is one of the weaknesses of this study. Also, not all the studies used a uniform duration of the PRF procedure; Lee et al. (6) used a duration of 240 seconds in PRF interventions (6), while De et al. (11) used a duration of 180 seconds. The PRF procedure is the manual procedure from Gauci (19), where denervation is carried out by heating at 42°C for 120 seconds in two cycles (19, 20).
In this study, it was found that PRF was effective in reducing the radicular pain score in lumbar HNP 12 weeks after the treatment. Chang (21) found that PRF effectively reduced the intensity of chronic neuropathic pain, such as radicular pain in HNP, post-herpetic neuralgia, and occipital neuralgia.
Side effects were evaluated in six studies included in this systematic review. According to the results, only one of the studies reported one subject who experienced an increase in radicular pain after PRF intervention and then withdrew from the study (6), and the rest reported no side effects during and after PRF (7-11).
Facchini et al. (22), in their systematic review of the PRF effectiveness in various conditions, found that complications from PRF interventions were infrequent. Most of the side effects, such as local swelling, pain at the needle injection site, and pain in the extremities, are brief and disappear independently. More serious complications include nerve trauma, vein injection, hematoma formation, and sciatic nerve injury. Infectious complications, including spondylodiscitis, intraarticular abscess, systemic infection, and even meningitis, have been reported. Moreover, mild complications, such as dizziness, flushing, sweating, nausea, hypotension, and syncope, have been reported (22, 23).
5. Conclusions
The PRF effect was not significant in reducing radicular pain scores due to lumbar HNP compared to the controls four and eight weeks after the treatment. However, PRF had a significant effect in lowering the radicular pain score 12 weeks after the treatment. PRF is relatively safe and has minimal side effects. It can be used as a promising clinical recommendation for pain management with minimally invasive techniques for radicular pain due to lumbar HNP.