1. Background
Osteoarthritis is the most common disease of synovial joints and the main cause of disability in the elderly. There is an increasing trend in osteoarthritis prevalence rate due to increased elderly population (1). Articular pain due to knee osteoarthritis (KOA) is the main cause of disability and activity restrictions and treatment-seeking, especially in elderly people (2). Pain management is the main therapeutic goal to achieve better joint function (3).
Non-invasive [e.g., physiotherapy (PT), TDCS (4), etc.] and invasive treatment options for KOA have been investigated. Platelet-rich plasma (PRP) (5, 6), prolotherapy (7, 8) have currently shown ideal outcomes. Hyaluronic acid, PRP, and prolotherapy require multiple injections that increase the risk of joint infections (9, 10). The next therapeutic step, but not in very young or aged subjects, is total knee arthroplasty, especially in recalcitrant pain (11). Pathologic assessments show osteophyte formation, subchondral changes, bone marrow edema, and articular surface destructions that decrease the joint space and stability beside soft tissue alterations such as synovial inflammation, capsular thickening, and ligament laxity (12).
Regarding peripheral sensitivity, intra-articular neurotoxins may be effective modalities (13, 14).
Botulinum toxin (BTX), produced by the bacterium Clostridium botulinum, acts on both sensory and motor neurons. Irreversible binding of BTX to presynaptic receptors of motor endplates inhibits acetylcholine release. This leads to the reduction of muscle activity and consequent muscle weakness (15). The BTX decreases the production of substance P and other pain generator substances by attaching to C fibers (16). BTX intra-articular injection is proposed to manage resistant joint pains (12). Although some primary studies showed some encouraging results for botulinum toxins, there were controversial results by other studies (17). Studies have shown better improvement in pain and function scores in short term, but there is a lack of evidence for long-term efficacy of BTX (18). According to different methods of physiotherapy interventions in KOA, modalities like local heat, TENS and pulsed ultrasound besides exercise therapy are effective in pain reduction and function of KOA patients (19-22).
2. Objectives
In this study, the long-term therapeutic effects of intra-articular were compared in the BTX and PT groups in patients with KOA.
3. Methods
3.1. Design and Setting
The original study was registered with the registration number IRCT20181217042028N2 at the Iranian Registry of Clinical Trials. We performed a single-blind randomized clinical trial from June 2018 for 9 months. The study was conducted in the Department of Physical Medicine and Rehabilitation at the Imam Reza Hospital.
3.2. Ethical Considerations
This study was approved by the Ethics Committee of Institutional Review Board of Aja University of Medical Sciences with the number of IR.AJAUMS.REC.1397.012. The researchers clarified all possible side effects, and informed consent forms were signed by the participants. The patients were free to withdraw from the study at any time.
3.3. Eligibility and Recruitment
The patients between 30 and 70 years of age who met the American College of Rheumatology criteria for KOA were recruited (23). Those with knee pain for more than 3 months, morning stiffness less than 30 min and joint crepitus were included in the study (24).
The patients with a history of diseases affecting knee joints like rheumatoid arthritis and gout and neuromuscular diseases were excluded from the study. Previous intra-articular injections, history of knee joint surgery, and trauma were the other exclusion criteria. Contraindications to intra-articular injection e.g., sepsis, intra-articular infections, intra-articular fracture, or uncontrolled coagulopathy were also considered the exclusion criteria (25).
All potential participants were evaluated based on the signs and symptoms of KOA. A standing lateral, anteroposterior and patellar view radiographs were taken. The study protocol was explained to all participants during the initial interview and after signing the written informed consent, the participants were allocated to one of the study groups.
3.4. Interventions
In the BTX group, the botulinum toxin was injected as a single intra-articular dose of 100 units (250 units from disport brand). The solution was diluted with 5 milliliters of normal saline and, after initial aspiration, was injected into the medial or lateral patellar tendon by a trained physician. In the PT group, exercise therapy besides modalities such as TENS (80 - 100 Hz, 100 - 200 milliseconds for 20 minutes), pulsed ultrasound (5: 1, 0.8 - 1.5 w/cm2 for five minutes), and superficial heat were used. Knee isometric exercises for quadriceps strengthening and calf/hamstring muscle stretching exercises were trained.
3.5. Outcome Measures
Pain as the primary outcome was evaluated with visual analog scale (VAS) in which 0 representing no pain and 10 showed the most severe pain. The Knee injury and Osteoarthritis Outcome Score (KOOS) was the secondary outcome. The KOOS is a self-report questionnaire and includes questions about pain, symptoms, activities during daily living, sport and recreational (Sport/Rec) activities, and quality of life (QOL). A normalized score from zero (extreme symptoms) to 100 (no symptoms) is given to each question. Salavati et al. validated the Persian version of KOOS showed that this version was culturally adapted, reliable, and valid (26). All measurements were performed at baseline and repeated at 1, 3, and 6 months after the intervention. All possible side effects were evaluated at each session.
3.6. Randomization and Blinding
This was a single-blind randomized clinical trial, and only outcome assessors were blinded. We used block randomization to randomly allocate 25 participants to each group. Random numbers were generated using a computer sequence generator software. Allocation concealment was done by sealed envelopes.
3.7. Statistical Analyses
The results are presented as mean (SD). Shapiro-Wilk test was used to test the normality of variables. Homogeneity of variances was investigated by Levene’s test. Independent sample t-test was used to compare means between the two groups. We used repeated measures ANOVA to compare the trend of variable means through time. The level of significance was set at α = 0.05. All data were analyzed with SPSS version 26 for windows.
4. Results
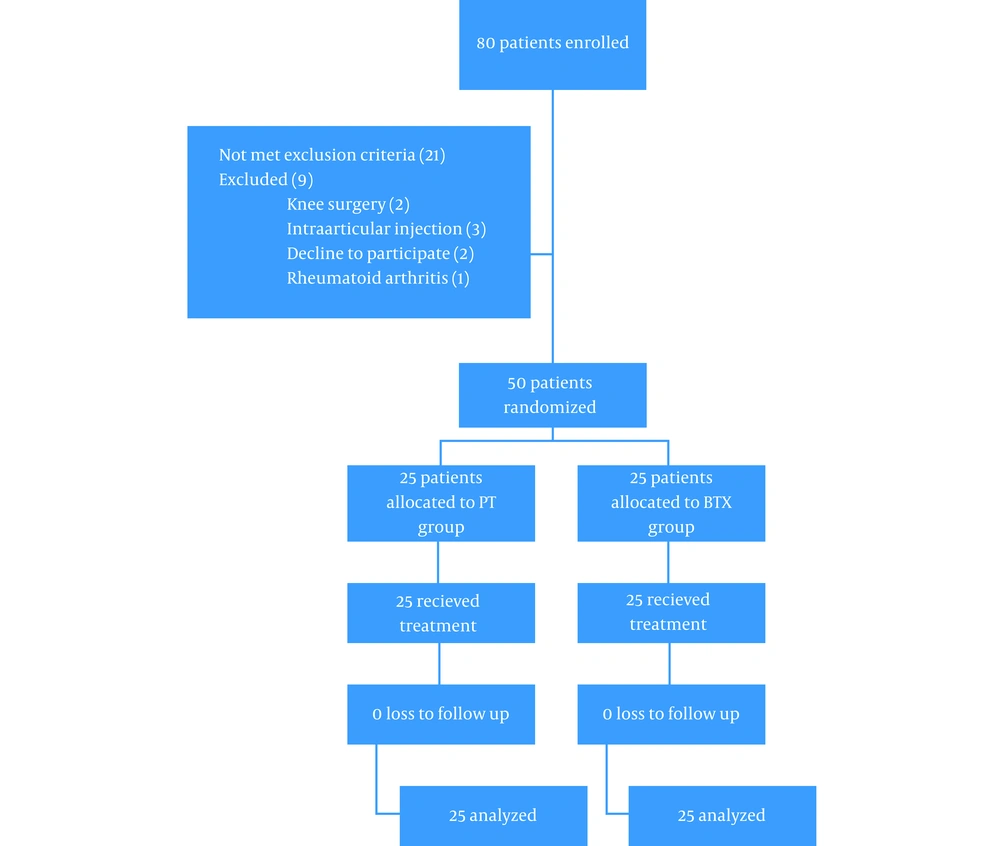
In this study, we enrolled 80 patients among whom 21 patients did not meet the inclusion criteria, and 9 patients were excluded, and overall, 50 patients randomized (Figure 1). All patients completed the study, and there was no loss to follow-up. In the BTX and PT groups, the subjects were female in 73 and 80%, respectively (P > 0.05). Table 1 compares baseline characteristics of the BTX and PT groups. The OA grade was 3 and 4 in 53 and 47% in the BTX group, respectively, and it was 3 and 4 in 60 and 40% in the PT group, respectively (P > 0.05). There was no significant difference between demographic data of the two groups, including age (77.7 ± 7.3 vs. 63.0 ± 8.0, P = 0.102) and BMI (31.3 ± 4.7 vs. 29.2 ± 3.5, P = 0.101). The VAS score was similar in the two groups at the beginning (6.67 ± 1.60 vs. 6.60 ± 1.84, P = 0.893). KOOS subscales like symptom, pain, ADL, and Sport/Rec activities did not significantly differ, but QOL was better in the BTX group than the PT group (86.2 ± 15 vs. 72.1 ± 11.5, P < 0.001). The only adverse effect in our study was severe pain in two cases in the BTX group that was improved by acetaminophen.
| Variables | BTX | PT | P-Value |
|---|---|---|---|
| Age b | 77.7 ± 7.3 | 63.0 ± 8.0 | 0.102 |
| Grade | 3.5 ± 0.5 | 3.5 ± 0.5 | 0.650 |
| Weight (kg) | 81.2 ± 12.2 | 76.4 ± 10.0 | 0.154 |
| Height (m) | 161 ± 7 | 161 ± 10 | 0.774 |
| BMI (kg/m2) | 31.3 ± 4.7 | 29.2 ± 3.5 | 0.101 |
| Sex c (%) | 0.425 | ||
| Male | 27 | 20 | |
| Female | 73 | 80 | |
| VAS | 6.67 ± 1.60 | 6.60 ± 1.84 | 0.893 |
| KOOS | |||
| Symptoms | 58.21 ± 23.58 | 53.92 ± 10.86 | 0.452 |
| Pain | 38.88 ± 18.22 | 44.02 ± 12.69 | 0.279 |
| ADL | 34.36 ± 13.29 | 42.20 ± 12.53 | 0.052 |
| Sports/Rec | 9.83 ± 19.14 | 12.75 ± 13.71 | 0.560 |
| QOL | 13.75 ± 15.07 | 27.81 ± 11.55 | 0.001 d |
a Values are expressed as mean ± SD unless otherwise indicated.
b Independent sample t-test.
c Fisher’s exact test.
d Significance at the level of 0.05.
Table 2 compares the difference between post-intervention and baseline measurements between the two groups by independent sample t-test. As shown in Table 2, one month after the intervention, all KOOS subscales were improved in the BTX group in comparison to the PT group (P < 0.001). This difference was statistically significant in the 3rd (P < 0.001 in all comparisons except Sport/Rec subscale in which P = 0.02) and 6th months (P < 0.001) after the intervention, and the improvement in all KOOS subscales and VAS score were higher in the BTX group than the PT group.
| Variables | Pain (VAS) | KOOS | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Symptoms | Pain | ADL | Sport/Rec | QOL | ||||||||
| 1 month - baseline | ||||||||||||
| BTX | -3.1 | 1.2 | 26.3 | 17.5 | 40.8 | 13.1 | 39.2 | 10.6 | 34.0 | 13.7 | 37.9 | 15.1 |
| PT | -1.3 | 1.4 | 5.1 | 12.3 | 2.0 | 12.5 | 3.0 | 12.7 | 10.0 | 11.3 | 4.3 | 9.5 |
| Significant b | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | ||||||
| 3 month - baseline | ||||||||||||
| BTX | -3.7 | 1.3 | 28.8 | 19.0 | 43.7 | 13.6 | 40.8 | 10.3 | 34.0 | 13.7 | 38.1 | 14.8 |
| PT | -1.8 | 1.4 | 5.8 | 16.7 | 6.3 | 19.2 | 2.8 | 10.5 | 19.2 | 29.7 | 10.9 | 23.8 |
| Significant | < 0.001 | < 0.001 | < 0.001 | < 0.001 | 0.02 | < 0.001 | ||||||
| 6 month - baseline | ||||||||||||
| BTX | -3.9 | 1.3 | 29.3 | 19.4 | 43.5 | 12.7 | 40.5 | 10.4 | 30.7 | 14.2 | 37.5 | 15.6 |
| PT | -1.5 | 1.0 | 0.5 | 11.8 | 0.1 | 13.0 | 2.8 | 10.5 | 10.2 | 11.8 | 3.8 | 9.8 |
| Significant | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | ||||||
a All measurements are stated as mean difference ± standard deviation.
b Between-group analysis was done with independent sample t-test (significance at the level of 0.05).
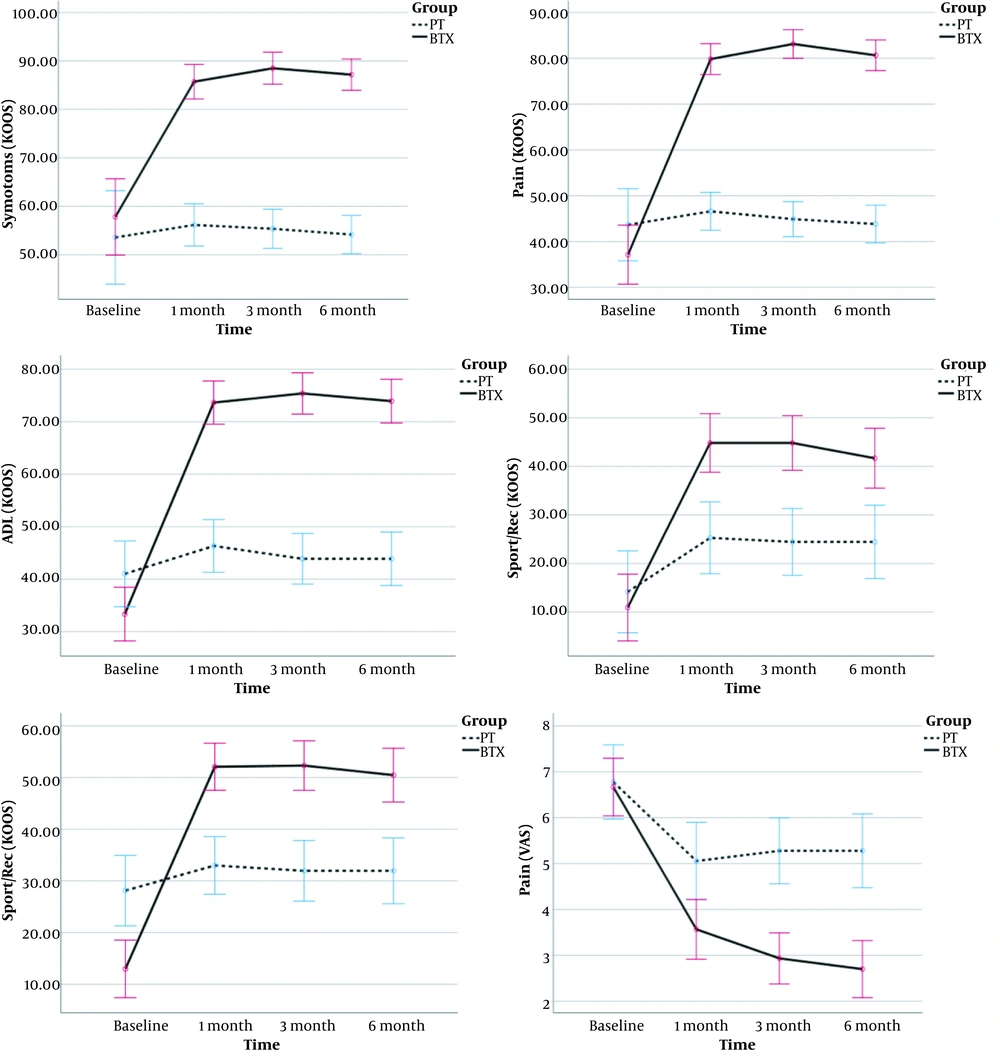
Figure 2 shows the trend of improvement in KOOS subscales and VAS score, which is calculated by within-group analysis of repeated-measurement ANOVA (RMANOVA). The trend of KOOS subscales and VAS score were improved over time in the BTX group (P < 0.001 in all tests), but the PT group showed no improvement (P > 0.05 except for Sport/Rec and VAS (P < 0.001). In addition, the difference was significant between the two groups in the 1st, 3rd, and 6th months of the intervention, and the BTX group had higher levels of improvement than the PT group. Although QOL was worse in the BTX group at the beginning, it has higher scores in the following investigations. The mixed ANOVA analysis compared the two groups in terms of pain (VAS). The assumption of sphericity was violated for the pain (Muchly's W for pain = 0.71, P = 0.009). Therefore, Greenhouse-Geisser correction was used for the degrees of freedom; epsilon pain = 0.83. The interaction effect of group × time were significant [F (2.4, 16.8) = 17.7, P < 0.001] and the effect size (partial Eta square) was 0.278.
5. Discussion
One of the main challenges in the management of KOA is cases with high-severity disease. The intra-articular injections may impose some costs and some possible adverse effects and need multiple injections for better persistent outcomes and may be effective only in low grades (27, 28). BTX injection is a novel therapeutic method for resistant painful conditions. Regarding small studies in this era and uncertainty about appropriate dose, BTX injection is not a conventional method (29). In this study, long-term efficacy of PT versus BTX injection was assessed.
The results of our study showed higher efficacy for BTX versus PT in short and long term. In our study, the VAS score was significantly lower after treatment in both groups, but the difference was statistically significant between the two groups after the intervention. Also, these results were seen for knee function according to KOOS subscales. The study conducted by Bao et al. (30) showed better efficacy for exercise plus botulinum toxin versus hyaluronate and normal saline (control group). However, Mendes et al. showed that hyaluronic acid has higher effectiveness than botulinum toxin in short-term (4 weeks) follow-up (18). In Mendes study, both groups showed improvement in VAS score and Western Ontario and McMaster Universities Osteoarthritis Index, but this improvement was higher in hyaluronate group.
The long-term efficacy of botulinum toxin has controversial results. A study done by Sun (31) showed no significant difference in six-month follow-up between botulinum toxin and hyaluronate plus exercise in ankle osteoarthritis. Our study showed that in the long term, both pain and function in KOA were improved in the BTX group versus the PT group. The difference seen in other studies may be due to lower osteoarthritis grades. There is no evidence of botulinum toxin effects on inflammatory pain in human studies (32). The study by Singh (33) on BTX-A resulted in better function and less articular stiffness, and the WOMAC score was reduced. Better ADL and quality of life in our study showed better performance and function of the knee in patients.
The mechanism of BTX to reduce pain in KOA is not well known. It is shown that substances like serotonin, prostaglandins, bradykinin and histamine have nociception activity on free nerve endings. It has been reported in the rat models that joint damage or inflammation caused by KOA could result in the production of various substances (e.g., prostaglandins, histamine, and serotonin) and then activating C- and A-delta fibers in peripheral articular tissue (34). Sensitizations of damaged joint tissue result in increased pain, and this pain is difficult to control with conventional therapy (35). Lately, it is found that BTX-A is capable of blocking central and peripheral sensitizations by inhibiting neurotransmitter release (36). Also, other studies showed that BTX-A might have an antinociceptive effect by downregulation of the expression of voltage-gated sodium channel on rat models (37). Accordingly, a plausible explanation for pain inhibition of BTX-A is reducing neurotransmitter release such as substance P, etc., thus blocking the pain signal pathway.
Muscular weakness is the most common adverse effect due to botulinum toxin injection, especially in cervical dystopia cases. Other side effects include arrhythmia, dysphagia, anaphylactic shock, skin rashes, and flu-like syndrome. None of these were seen in our study. As mentioned, the only adverse effect in our study was severe pain in two cases that was improved by acetaminophen. This is due to high volume injection in the joint with severe OA that is destructed with high sensation status. Also, the intra-articular versus systemic injection may be used as a safe method. Also, there were no adverse effects in the PT group.
Totally, it is concluded that the use of BTX can reduce pain and improve the function and quality of life in patients with high-severity KOA. However, further dose-finding and safety studies with larger sample size are required to get more definite applicable results.
5.1. Conclusion
It is hypothesized that botulinum toxin can reduce neurotransmitter release; thus blocking the pain signal pathway. In this study, we concluded that the use of BTX can reduce pain and improve the function and quality of life in patients with KOA. This improvement was significant in long-term follow-up in the BTX group even in 6 months after the intervention. We can conclude that BTX can be a suitable long-term treatment option for KOA, even in high grades arthritis.